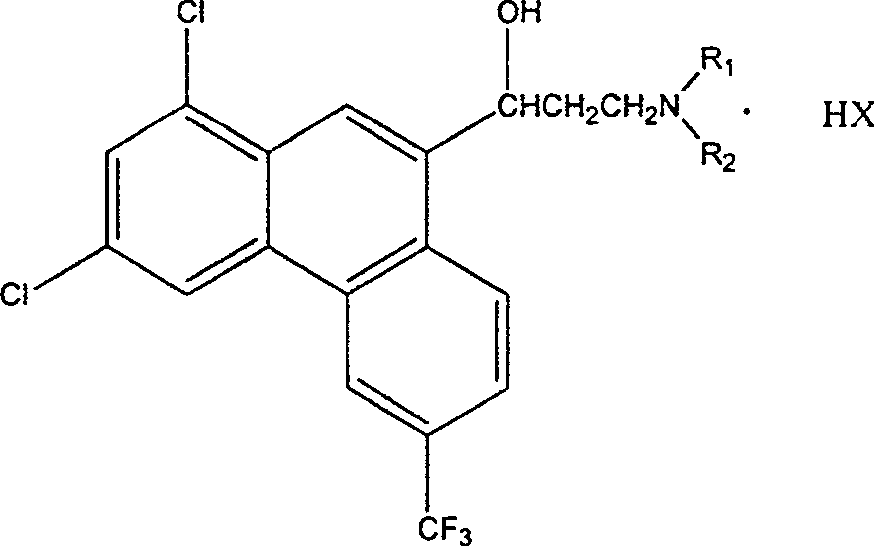
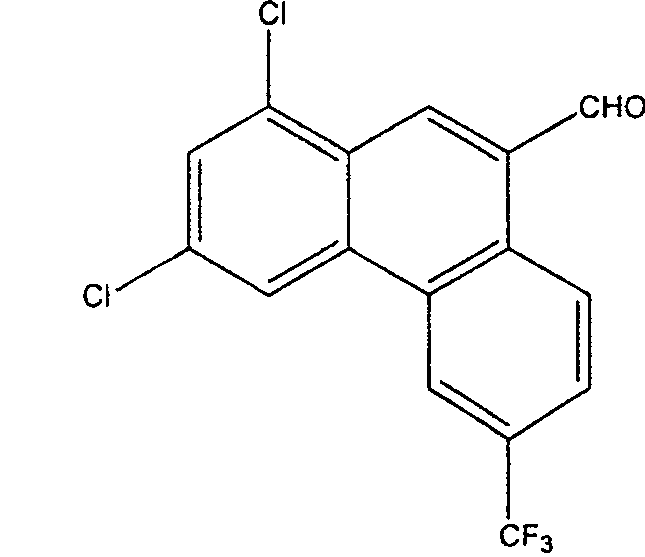
Method for synthesizing 1,3-dichloro-6-trifluoromethyl-phenanthrene-9-formaldehyde
A trifluoromethyl, synthesis method technology, applied in the field of synthesis of 1,3-dichloro-6-trifluoromethyl-9-phenanthrene formaldehyde, can solve the problems of harsh conditions, low melting point, high anhydrous requirements, etc. Achieve the effects of mild and easy-to-control reaction conditions, simple process operation, and low anhydrous requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 34.7 grams of 1,3-dichloro-6-trifluoromethyl-9-phenanthrene methanol (0.10 moles) and 800 milliliters of toluene to a 1000-milliliter four-neck flask with mechanical stirring, a thermometer, and a reflux condenser, and add 0.3 g cuprous chloride (3 mmol) and 0.6 g 1,10-phenanthroline (3 mmol), add 0.53 g diethyl hydrazine dicarboxylate (3 mmol) and 27.6 g potassium carbonate (0.20 mole) , stirring to raise the temperature, and slowly inject air at 60° C. for bubbling reaction for 40 hours. After the reaction is complete, filter, wash the filter cake with 3×100 ml of toluene, combine the toluene, concentrate the toluene under reduced pressure to 100 ml, cool to below 20°C, and precipitate the product 1,3-dichloro-6-trifluoromethyl-9 -Phenanthrene formaldehyde was filtered and dried to obtain a yellowish white solid, which was dried to obtain 28.0 g of the product with a yield of 81.1%. Melting point 185.5~185.8℃, content 99.5% (HPLC).
Embodiment 2
[0021] The operation of Example 1 was repeated, only the cuprous chloride in Example 1 was changed to 0.4 gram of cuprous bromide (2.8 mmol), and the reaction time was 30 hours to obtain a product yield of 80.5%.
Embodiment 3
[0023] The operation of Example 1 was repeated, the reaction temperature in Example 1 was increased to 80° C., and the reaction time was 20 hours to obtain a product yield of 79.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com