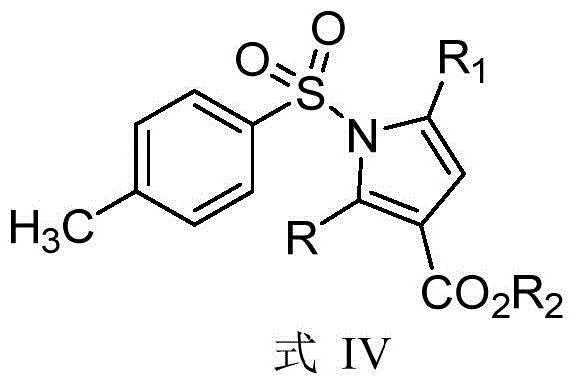
Synthetic method of dihydropyrrole and pyrrole compounds
A technology of dihydropyrrole and synthesis method, applied in the direction of organic chemistry and the like, achieves the effects of high atom economy, environmental friendliness and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
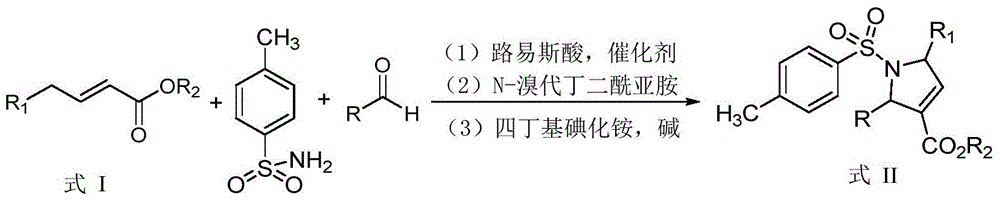
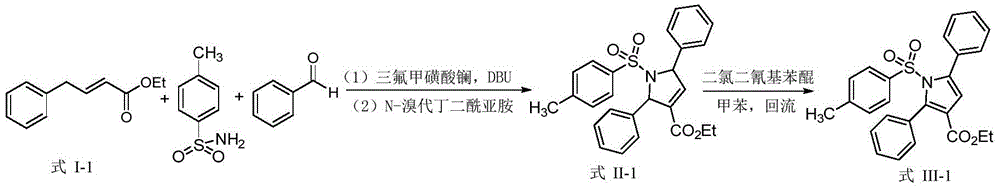
[0021] Taking the synthesis of the dihydropyrrole compound shown in III-1 and the pyrrole compound shown in IV-1 as an example, its synthetic route and specific synthetic method are as follows:
[0022]
[0023] Add 87.9mg (0.462mmol) α, β-unsaturated ester shown in formula I-1, 79.1mg (0.462mmol) p-toluenesulfonamide, 5.4mg (0.00924mmol) trifluoromethanesulfonate successively in 10mL round bottom flask Lanthanum acid, 14.1mg (0.0925mmol) DBU, 49.0mg (0.462mmol) benzaldehyde, 0.25mL isopropanol, stirred at room temperature for 12 hours, then added 164.5mg (0.924mmol) NBS, stirred at room temperature for 2 hours, then added 51.2 mg (0.1386mmol) TBAI and 1.386mL1mmol / L potassium carbonate aqueous solution, heated to reflux for 3 hours to stop the reaction, cooled the reaction system to room temperature, filtered under reduced pressure, washed the solid with a small amount of dichloromethane several times, and combined the filtrate , and pour it into water, let stand to separa...
Embodiment 2
[0030] Taking the synthesis of the dihydropyrrole compound shown in III-2 and the pyrrole compound shown in IV-2 as an example, its synthetic route and specific synthetic method are as follows:
[0031]
[0032] In Example 1, the benzaldehyde used was replaced with equimolar p-tolualdehyde, and the other steps were the same as in Example 1 to obtain a white solid 4,5-dihydro-2-phenyl-5-(4-methanol Base) phenyl-1-(4-methylphenyl)sulfonyl-4-pyrrole-α-ethyl acetate (dihydropyrrole compound shown in formula III-2), its yield is 68%, structure The characterization data are as follows:
[0033] 1 HNMR (300MHz, CDCl 3 )δ: 7.29-7.35 (m, 4H, Ph), 7.15 (d, J = 8.1Hz, 2H, Ph), 7.03 (d, J = 7.8Hz, 2H, Ph), 6.91-6.99 (m, 5H, Ph), 6.66 (t, J=1.5Hz, 1H, CH), 5.91 (dd, J=5.4, 0.6Hz, 1H, CH), 5.84 (dd, J=5.4, 1.8Hz, 1H, CH), 3.96 -4.14(m, 2H, CH 2 ), 2.35(s, 3H, CH 3 ), 2.34 (s, 3H, CH 3 ), 1.12(t, J=7.2Hz, 3H, CH 3 ).
[0034] 13 CNMR (100MHz, CDCl 3 )δ: 162.01, 142.15, 139.12, ...
Embodiment 3
[0039] Taking the synthesis of the dihydropyrrole compound shown in III-3 and the pyrrole compound shown in IV-3 as an example, its synthetic route and specific synthetic method are as follows:
[0040]
[0041] In Example 1, the benzaldehyde used was replaced with equimolar p-methoxybenzaldehyde, and the other steps were the same as in Example 1 to obtain a white solid 4,5-dihydro-2-phenyl-5-(4- Methoxy) phenyl-1-(4-methylphenyl) sulfonyl-4-pyrrole-α-ethyl acetate (dihydropyrrole compound shown in formula III-3), its yield is 62% , the structural characterization data are as follows:
[0042] 1 HNMR (300MHz, CDCl 3 )δ: 7.29-7.41 (m, 5H, Ph), 7.16 (d, J = 8.4Hz, 2H, Ph), 6.93-7.01 (m, 4H, Ph), 6.75 (d, J = 8.4Hz, 2H, Ph), 6.66 (bs, 1H, CH), 5.93 (d, J=5.4Hz, 1H, CH), 5.82 (d, J=5.4Hz, 1H, CH), 3.99-4.14 (m, 2H, CH 2 ), 3.82 (s, 3H, OCH 3 ), 2.34 (s, 3H, CH 3 ), 1.12(t, J=7.2Hz, 3H, CH 3 ).
[0043] 13 CNMR (100Hz, CDCl 3 )δ: 162.03, 159.47, 142.17, 139.06, 138.36,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com