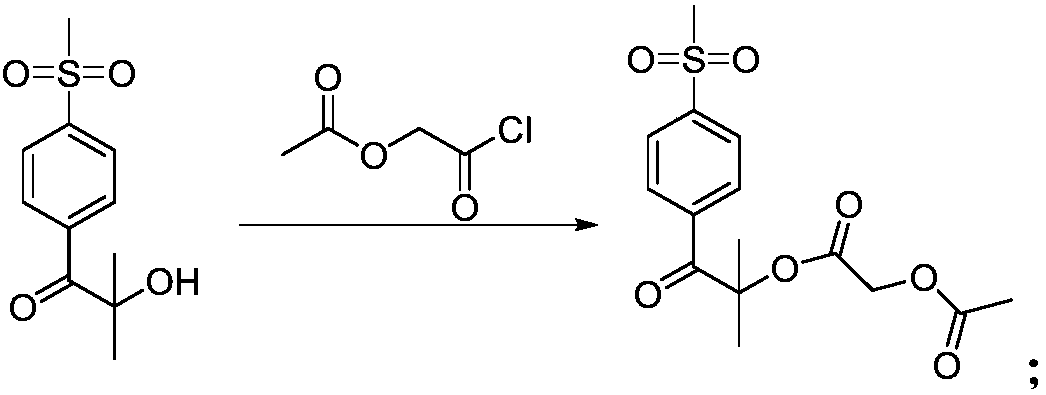
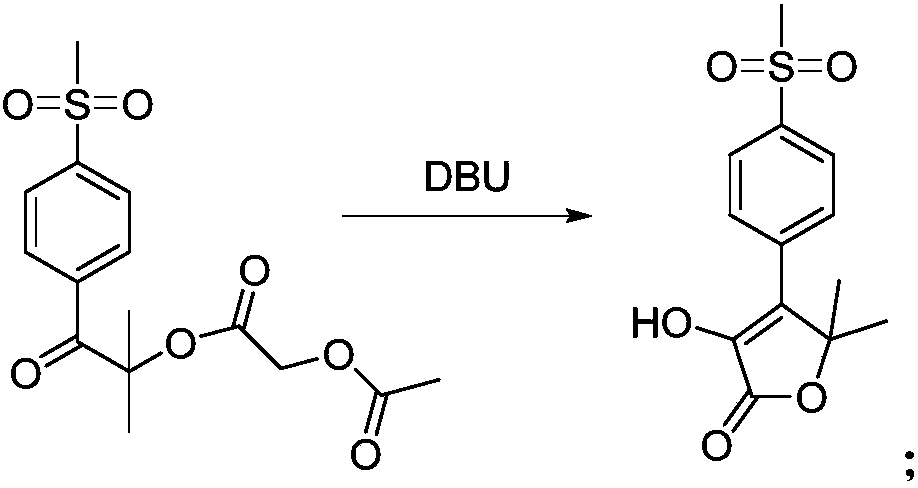
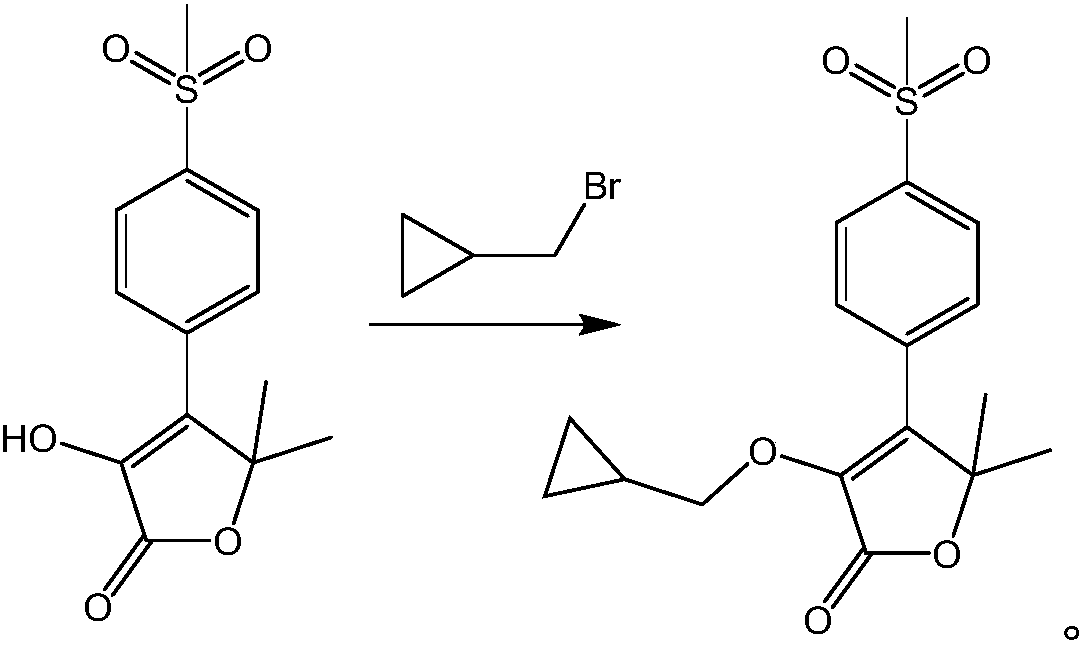
Synthesis methods of firocoxib and firocoxib intermediate
A firocoxib and synthetic method technology, applied in the field of drug synthesis, can solve problems such as not suitable for large-scale industrial production, unfavorable for the environment and occupational health, strong toxicity and corrosiveness, etc. Improved production efficiency and yield, environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] Specifically, the method for synthesizing Ferocoxib intermediate includes the following steps:
[0045] S1: Using p-bromopropiophenone as a starting material, methylating the p-bromopropiophenone and a methylating reagent to obtain the first intermediate A1.
[0046] Specifically, p-bromopropiophenone is dissolved in the first solvent, a strong base and a methylating reagent are added, ethyl acetate and water are added for liquid separation after the reaction is complete, the organic phase is dried over anhydrous sodium sulfate, and the solvent is evaporated under reduced pressure The first intermediate A1 was obtained.
[0047] The embodiment of the present invention uses p-bromopropiophenone as the starting material to generate the first intermediate A1 through the methylation reaction. The methylation reaction is carried out at room temperature under mild reaction conditions and avoids the use of environmentally unfriendly thioethers as starting materials. Starting materia...
Embodiment 1
[0069] Example 1 Preparation of 1-(4-bromophenyl)-2-methylacetone
[0070] This embodiment provides a method for preparing the first intermediate A1, which takes p-bromopropiophenone as a starting material, and performs a methylation reaction between p-bromopropiophenone and a methylating reagent to obtain the first intermediate A1. The preparation steps are as follows:
[0071] Dissolve p-bromopropiophenone (100g, 1eq) in 400mL dimethylformamide at room temperature, add sodium hydroxide (56.3g, 3eq) and methyl iodide (133.3g, 2eq), and stir overnight at room temperature. After the reaction is complete, add 500 mL ethyl acetate and 500 mL water, extract and separate the layers, the organic phase is dried over anhydrous sodium sulfate, and the solvent is evaporated under reduced pressure to obtain the first intermediate A1, namely: 1-(4-bromophenyl)-2- Methyl acetone, (110g, light yellow liquid).
Embodiment 2
[0072] Example 2 Preparation of 1-(4-bromophenyl)-2-methylacetone
[0073] This embodiment provides a method for preparing the first intermediate A1, which takes p-bromopropiophenone as a starting material, and performs a methylation reaction between p-bromopropiophenone and a methylating reagent to obtain the first intermediate A1. The preparation steps are as follows:
[0074] Dissolve p-bromopropiophenone (5.0 g, 1 eq) in 20 mL DMSO at room temperature, and add lithium hydroxide (2.0 g, 3.7 eq). After stirring until the solid is dissolved, methyl iodide (5.0 g, 1.5 eq) is added dropwise, and the mixture is stirred at room temperature overnight. After the reaction is complete, add 30 mL of ethyl acetate and 30 mL of water, extract and separate the layers, dry the organic phase over anhydrous sodium sulfate, and distill off the solvent under reduced pressure to obtain intermediate A1, namely: 1-(4-bromophenyl)-2-methyl Acetone, (5.8g, light yellow liquid).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com