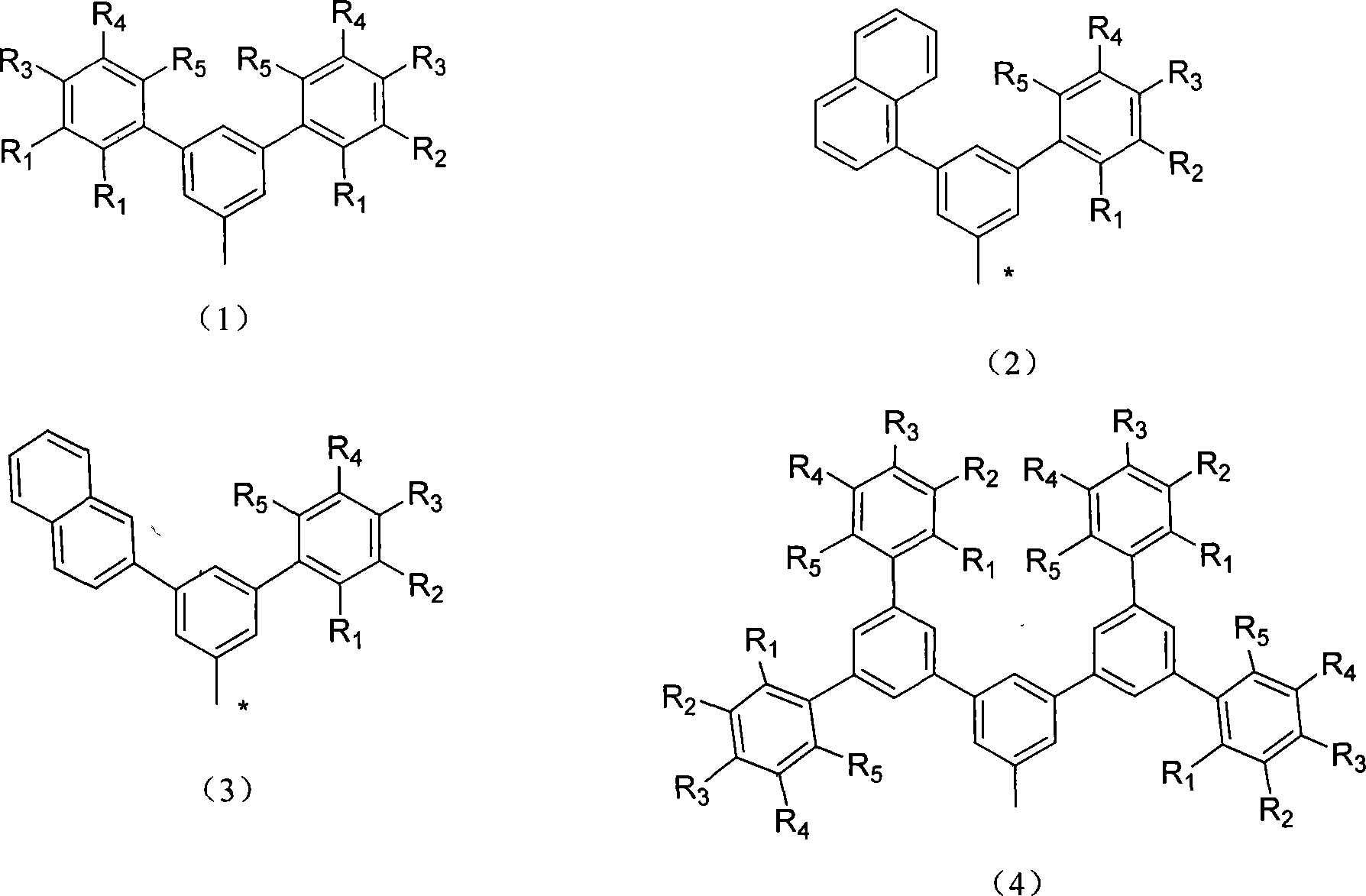
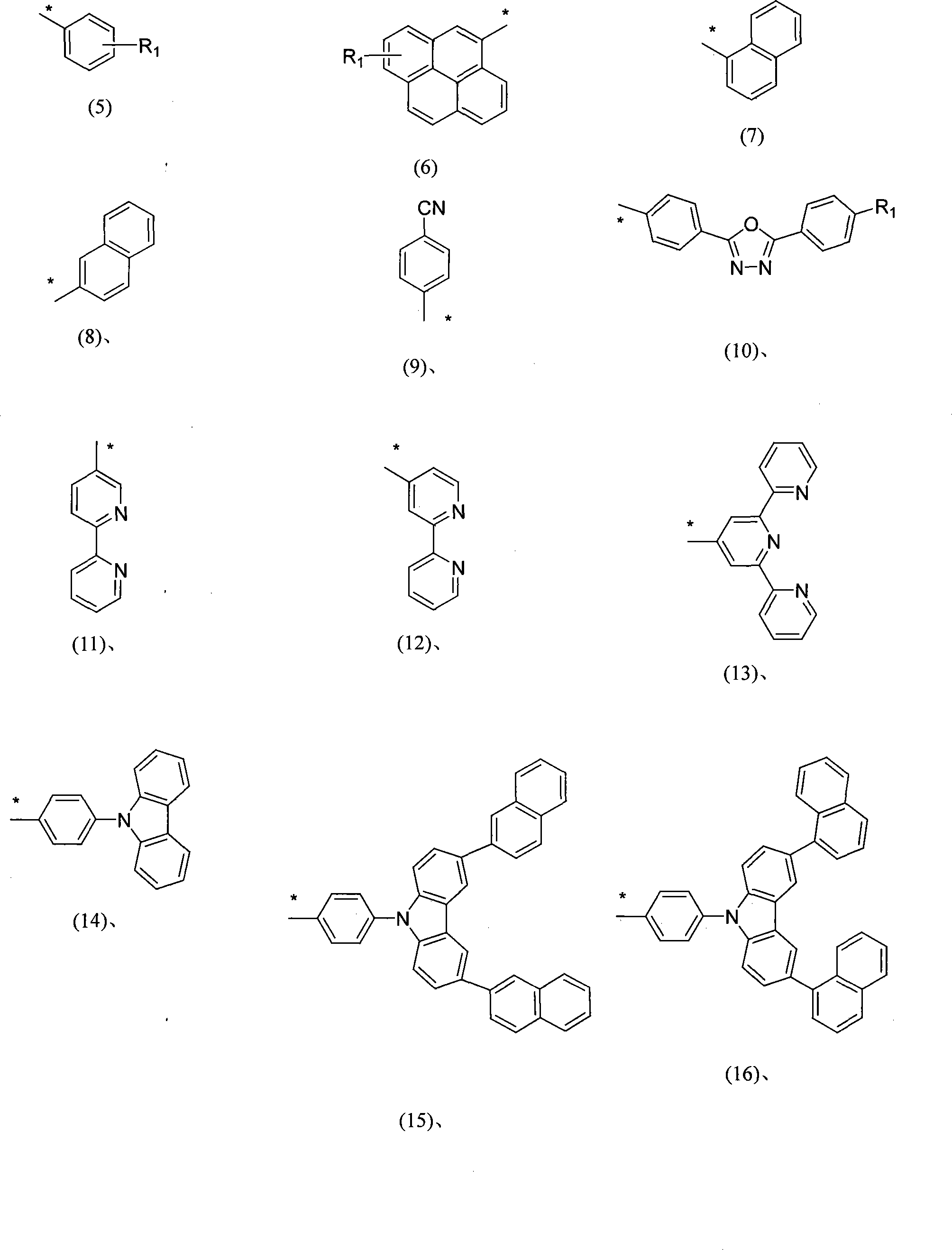
Soluble branch substituted anthracene molecule blue material as well as preparation method and uses thereof
A blue light material, soluble technology, applied in the direction of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., to achieve the effect of convenient purification, simple synthesis route and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1,2,4, the preparation of 6-tribromoiodobenzene:
[0033] The mechanism of the reaction is as follows:
[0034]
[0035] Pour 2,4,6-tribromoaniline (46.95g, 142mmol) into a three-necked flask under ice-salt bath conditions, measure concentrated sulfuric acid (13.95g, 7.62mL, 142mmol), and then quickly dropwise add it into the flask, Mechanical stirring. Weigh sodium nitrite (14.73g, 213mmol) and dissolve it in 100mL of distilled water, pour it into the dropping funnel, start to add it slowly when the temperature drops below -5°C, control the temperature not to exceed 5°C and stir for 1h after the addition is completed , Potassium iodide (47.25g, 282mmol) was dissolved in 100ml of distilled water, poured into the dropping funnel, temperature controlled at 0-5°C and slowly added dropwise with enhanced stirring, and stirred for 30min after the dropwise addition was completed. Pour the mixed liquid into 300mL of NaHSO 3 After stirring in aqueous solution, C...
Embodiment 2
[0036] Embodiment 2,3, the preparation of 5-two p-tert-butylphenylbromobenzene:
[0037] A 500 mL three-neck flask was exhausted with Ar for 30 min, then magnesium powder (11.1 g, 468.45 mmol), iodine crystals (3-4 grains), THF (200 mL) were added, and stirred. Dilute p-tert-butylbromobenzene (89.73 g, 416.4 mmol) with 50 mL of THF and pour it into a constant pressure dropping funnel, and protect it with Ar gas. Slowly add p-tert-butylbromobenzene dropwise to the flask, and heat the flask with a hair dryer until the brown color of the solution fades and boils. After the dropwise addition, the oil bath was refluxed for 2h to obtain the Grignard reagent. Then the Grignard reagent was transferred to a 1L three-necked flask, and 2,4,6-tribromoiodobenzene (45.87g, 104.1mmol) was added to a constant pressure dropping funnel and dissolved with 150mL of THF, then slowly added dropwise to in the flask. Continue to stir for 1h after the dropwise addition, and then heat to reflux for ...
Embodiment 3
[0041] Example 3, Preparation of 3,5-di-p-tert-butylphenyl-1-(4,4,5,5-tetramethyl-1,3,2-dioxaboryl)benzene:
[0042]
[0043] Dissolve 3,5-di-tert-butylphenyl-1-bromobenzene (4.21 g, 10 mmol) in 80 mL of dry tetrahydrofuran,
[0044] Protect with nitrogen gas, and cool to -78°C with liquid nitrogen / isopropanol. 2.5M n-BuLi (5.6 mL, 14 mmol) was slowly added dropwise into the reaction flask, and the mixture changed from colorless to yellow. After the dropwise addition, continue to stir at -78°C for 1 h, then add 4,4,5,5-tetramethyl-1,3,2-isopropanol borate (2.8 mL, 14 mmol) with a syringe, yellow Disappeared immediately, then naturally warmed to room temperature and stirred for 24h. The mixture was washed with distilled water and CH 2 Cl 2 Extracted 3 times, the oil layer was extracted with MgSO 4 dry.
[0045] A white solid was obtained by column chromatography.
PUM
| Property | Measurement | Unit |
|---|---|---|
| light emitting area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com