Affinity chromatography medium employing tetrapeptide as functional ligand and preparation method of affinity chromatography medium
A chromatographic medium and matrix technology, which is applied in the fields of protein chromatography separation, affinity chromatography medium and its preparation, can solve the problems of weak salt-resistant binding ability of ligands and affect antibody activity, etc., so as to facilitate antibody binding and cleaning The effect of convenient regeneration and stable medium properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
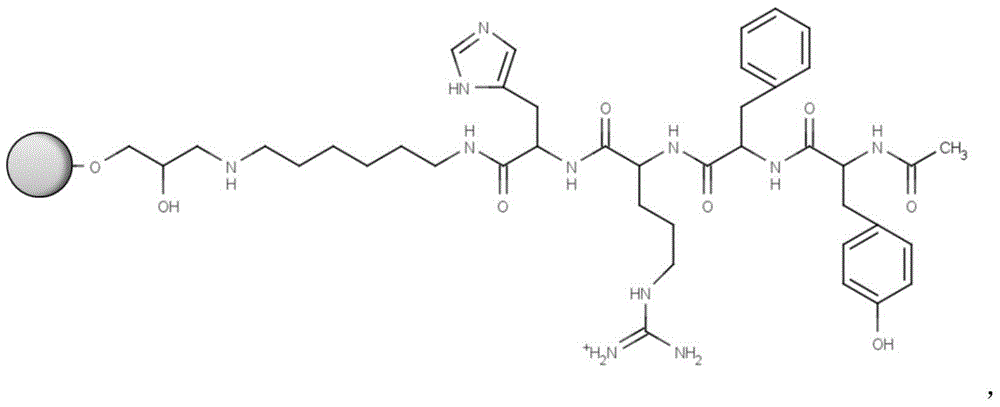
[0022] Take 10 g of drained agarose gel, add 10 mL of 20% (v / v) dimethyl sulfoxide solution, 10 mL of allyl bromide and 5 g of sodium hydroxide, activate in a shaker at 180 rpm at 30 ° C for 48 hours, and filter with suction. Wash with deionized water to obtain an activated chromatography matrix; then mix the activated matrix and 5g of N-bromosuccinimide for bromoalcoholization, react in a shaker at 180rpm at 30°C for 1 hour, filter with suction, and use deionized Wash with water; then mix the brominated matrix with 3mL of hexamethylenediamine and 1M sodium carbonate buffer (pH 12), react in a shaker at 180rpm at 30°C for 24 hours to obtain the amino-activated matrix; take 1g of the amino-activated matrix, and use Wash with deionized water, absolute ethanol and anhydrous N,N-dimethylformamide, filter with suction, add to 2mL containing 100mg tetrapeptide, 100mg 2-(7-azobenzotriazole)-N,N, N',N'-tetramethyluronium hexafluorophosphate and 62.5 μL of N,N-diisopropylethylamine in ...
Embodiment 2
[0024] Take 10 g of drained agarose gel, add 7.5 mL of 20% (v / v) dimethyl sulfoxide solution, 8 mL of allyl bromide and 4 g of sodium hydroxide, activate in a shaker at 180 rpm at 30 °C for 36 hours, and filter with suction , washed with deionized water to obtain an activated chromatography matrix; then the activated matrix and 4g N-bromosuccinimide were mixed for bromoalcoholization, reacted in a shaker at 180rpm at 30°C for 1 hour, filtered with suction, and used to remove Wash with ionic water; then mix the brominated matrix with 3mL of hexamethylenediamine and 1M sodium carbonate buffer (pH 11), react in a shaker at 180rpm at 30°C for 16 hours to obtain the amino-activated matrix; take 1g of the amino-activated matrix, and use Wash with deionized water, absolute ethanol and anhydrous N,N-dimethylformamide, filter with suction, add to 2mL containing 80mg tetrapeptide, 80mg 2-(7-azobenzotriazole)-N,N ,N',N'-tetramethyluronium hexafluorophosphate and 50 μL N,N-diisopropylethy...
Embodiment 3
[0026]Take 10 g of drained agarose gel, add 5 mL of 20% (v / v) dimethyl sulfoxide solution, 6 mL of allyl bromide and 3 g of sodium hydroxide, activate in a shaker at 180 rpm at 30 ° C for 24 hours, and filter with suction. Wash with deionized water to obtain an activated chromatography matrix; then mix the activated matrix and 3g of N-bromosuccinimide for bromoalcoholization, react in a shaker at 180rpm at 30°C for 1 hour, filter with suction, and use deionized Wash with water; then mix the brominated matrix with 1.8mL hexamethylenediamine and 1M sodium carbonate buffer (pH 11), react in a shaker at 180rpm at 30°C for 12 hours to obtain the amino-activated matrix; take 1g of the amino-activated matrix, and use Wash with deionized water, absolute ethanol and anhydrous N,N-dimethylformamide, filter with suction, add to 2mL containing 60mg tetrapeptide, 60mg 2-(7-azobenzotriazole)-N,N ,N',N'-tetramethyluronium hexafluorophosphate and 37.5 μL N,N-diisopropylethylamine in N,N-dimet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Saturated adsorption capacity | aaaaa | aaaaa |
| Dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com