Synthesis method of N-phenyl-3(4-bromophenyl) carbazole
A synthesis method and technology of phenylcarbazole, which are applied in the synthesis field of N-phenyl-3-(4-bromophenyl)carbazole, can solve the problem of frostbite of operators, increased risk factor of reaction kettle and waste of copper powder and other problems, to achieve the effect of reducing production equipment requirements, improving production safety environment, and reducing waste discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
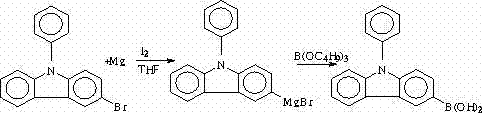
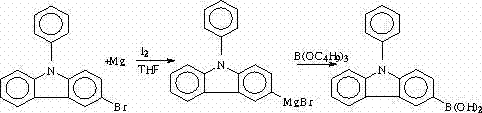
[0044] Example, a synthetic method of N-phenyl-3-(4-bromophenyl)carbazole, the synthetic route is as follows:
[0045] Reaction I
[0046]
[0047] Ⅰ
[0048] Reaction II
[0049]
[0050] Ⅱ
[0051] Reaction III
[0052]
[0053] Ⅲ
[0054] Reaction Ⅳ
[0055]
[0056]
[0057] Among them, I is N-phenylcarbazole, II is N-phenyl-3-bromocarbazole, III is N-phenyl-3-boronic acid carbazole, and IV is N-phenyl-3-(4-bromocarbazole). Phenyl) carbazole.
[0058] This implementation takes N-phenyl-3-(4-bromophenyl)carbazole, which is IV, as an example. The instruments used are GC-7820 gas chromatograph, Shimadzu LC-10ATvp high performance liquid chromatograph, and R201 Rotary evaporator, 2XZ-4 type rotary vane vacuum pump, DZ-1A type vacuum drying oven, SHB- Circulating water vacuum pump; reagents used: carbazole, iodobenzene, cuprous oxide, 1,10-phenanthroline, anhydrous potassium carbonate, silica gel, hydrochloric a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com