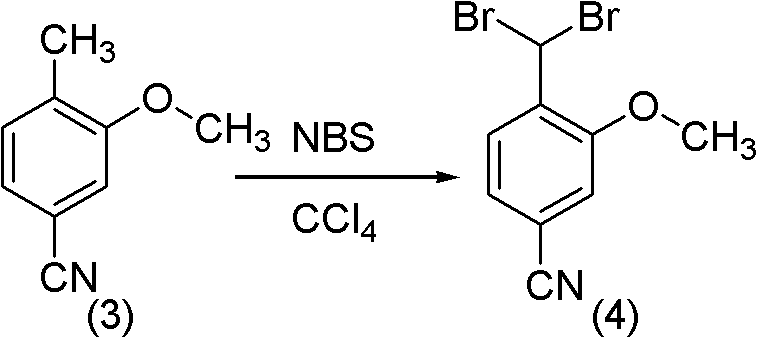
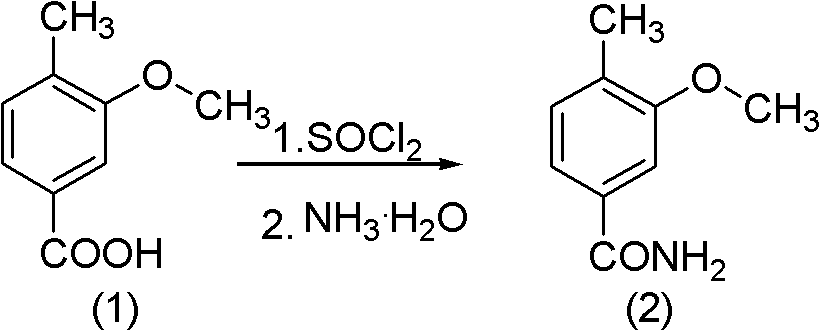Method for synthesizing 2-methoxy-4-cyano benzaldehyde
A synthesis method, a technology of methyl benzamide, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of high price of silver nitrate, unsuitable for industrial production, etc., and achieve easy operation and rapid reaction. , the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Synthesis of 3-methoxy-4-methylbenzamide: Add 900g (5.42mol) of 3-methoxy-4-methylbenzoic acid into a 2L four-necked reaction flask, add 496ml of thionyl chloride (6.83mol) stirring, began to slowly heat up to 80 ° C, the reaction liquid has a white slurry gradually turned into a brown liquid, and finally turned into a brownish black, about 2h after the reaction is completed, add 150ml of toluene to distill under reduced pressure, and distill off the remaining dichloro sulfoxide. Then the reaction solution is added to the beaker or reaction flask that 2.5L (7.3mol) of 25% ammoniacal liquor is housed, stirs while adding dropwise, there is pale yellow solid to separate out, suction filtration, infrared drying obtains 850g product (2), collects The rate is 95%.
[0019] (2) Synthesis of cyanide: Add 82.5 g (0.5 mol) of amide (2) into the 1L four-necked reaction flask, add 89.3 g (0.75 mol) of thionyl chloride dropwise into the reaction flask, and the dropwise addition...
Embodiment 2
[0023] (1) Synthesis of 3-methoxy-4-methylbenzamide: Add 900g (5.42mol) of 3-methoxy-4-methylbenzoic acid into a 2L four-necked reaction flask, add 496ml of thionyl chloride (6.83mol) stirring, began to slowly heat up to 90 ° C, the reaction liquid has a white slurry gradually turned into a brown liquid, and finally turned into brown black, about 2h after the reaction was completed, add 150ml of toluene to distill under reduced pressure, and distill off the remaining dichloro sulfoxide. The reaction solution is then added to a beaker or a reaction flask with 2.5L (7.3mol) of 25% ammonia, and stirred while adding dropwise, a pale yellow solid is precipitated, filtered by suction, and dried by infrared to obtain 769g of product (2). The rate is 86%.
Embodiment 3
[0025] Reaction (1) is the same with embodiment 1.
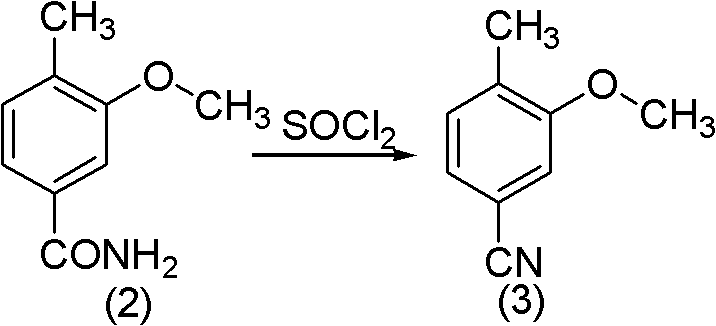
[0026] (2) Synthesis of cyanide: Add 82.5 g (0.5 mol) of amide (2) into the 1L four-necked reaction flask, add 89.3 g (0.75 mol) of thionyl chloride dropwise into the reaction flask, and the dropwise addition is completed in about 45 minutes. The temperature was controlled at 80°C, and the reaction was carried out for 0.5h. The reaction solution was poured into 800 g of ice water and stirred while pouring, a yellow solid was precipitated, filtered by suction, and dried to obtain 65 g of product (3), with a yield of 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com