Oligothiophene derivative and preparation method thereof
A technology of oligothiophene and derivatives, applied in chemical instruments and methods, color-changing fluorescent materials, organic chemistry, etc., can solve problems such as unstable color changes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
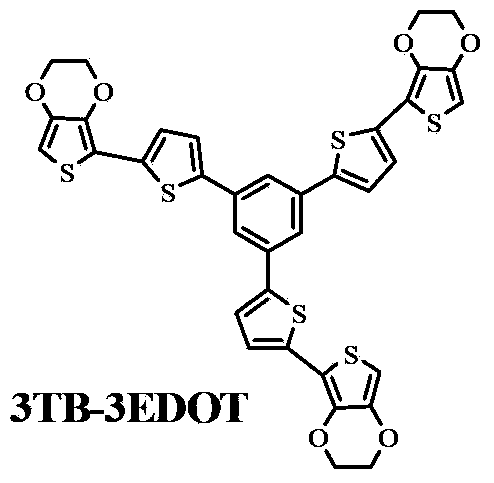
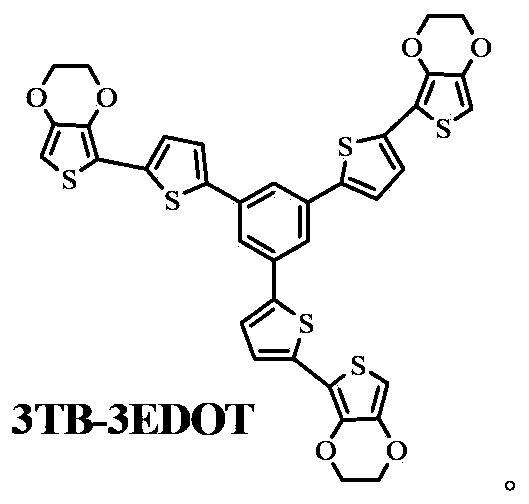
[0018] Example 1: Preparation of 1,3,5-tri[5',2"-(3",4"-dioxyethylene-thienyl)-2'-thienyl]benzene (3TB-3EDOT)
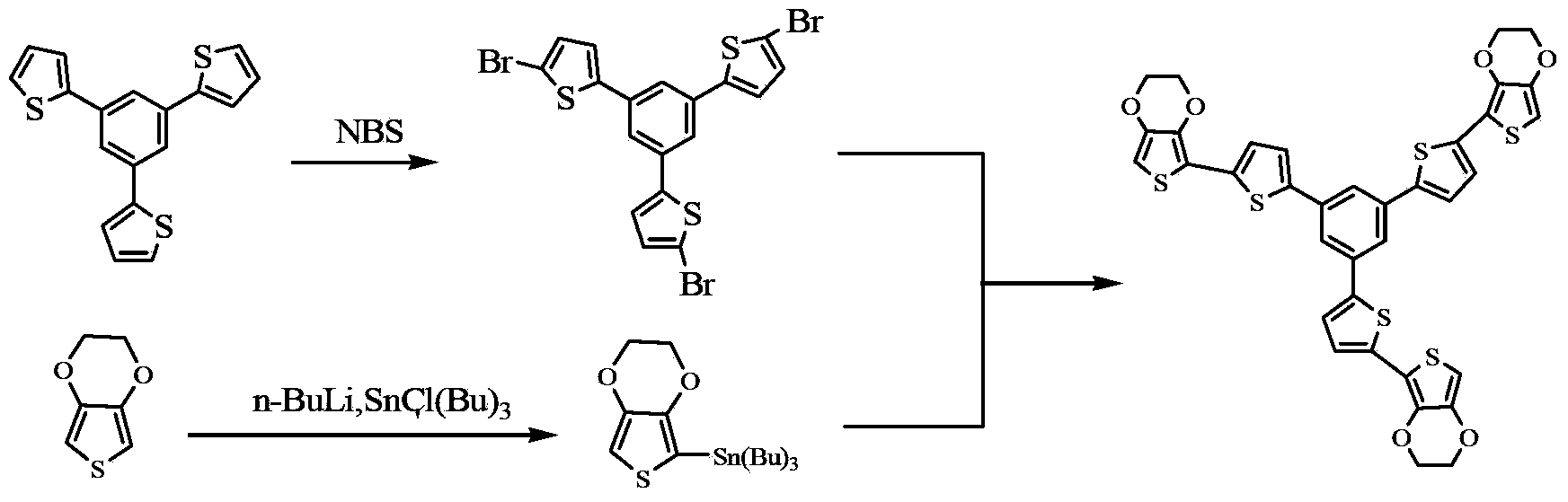
[0019] The first step is to add 1,3,5-tris(2′-thienyl)-benzene (3TB) into the Erlenmeyer flask, dissolve it with dichloromethane, and add N-bromosuccinimide under magnetic stirring (NBS), reacted at room temperature for 20 hours. The solvent was removed to give a crude product of 1,3,5-tris(5'-bromo-2'-thienyl)benzene (3TB-3Br). Using petroleum ether as a flushing liquid, the purer 3TB-3Br is obtained by separation with a chromatographic column, and then recrystallized to obtain pure 3TB-3Br.
[0020] In the second step, under the protection of nitrogen, 3,4-dioxyethylenethiophene (EDOT) and anhydrous tetrahydrofuran were added into a three-necked flask, and the reaction temperature was controlled at -78°C. After the temperature was stabilized, n-butyllithium was added dropwise, and after the dropwise addition, the temperature was raised to room temperature, and th...
Embodiment 2
[0027] Example 2: Preparation of 1,3,5-tris[5',2"-(3",4"-dioxyethylene-thienyl)-2'-thienyl]benzene (3TB-3EDOT)
[0028] The first step is to add 1,3,5-tris(2′-thienyl)-benzene (3TB) into the Erlenmeyer flask, dissolve it with dichloromethane, and slowly add N-bromosuccinyl under magnetic stirring imine (NBS), reacted at room temperature for 20 hours. The solvent was removed to give a crude product of 1,3,5-tris(5'-bromo-2'-thienyl)benzene (3TB-3Br). Using petroleum ether as a flushing liquid, the purer 3TB-3Br is obtained by separation with a chromatographic column, and then recrystallized to obtain pure 3TB-3Br.
[0029] In the second step, under the protection of nitrogen, 3,4-dioxyethylenethiophene (EDOT) and anhydrous tetrahydrofuran were added into a three-necked flask, and the reaction temperature was controlled at -78°C. After the temperature stabilized, n-butyllithium was slowly added dropwise, and after the dropwise addition, it was raised to room temperature, and t...
Embodiment 3
[0036] Example 3: Preparation of 1,3,5-tri[5',2"-(3",4"-dioxyethylene-thienyl)-2'-thienyl]benzene (3TB-3EDOT)
[0037]The first step is to add 1,3,5-tris(2′-thienyl)-benzene (3TB) into the Erlenmeyer flask, dissolve it with dichloromethane, and slowly add N-bromosuccinyl under magnetic stirring imine (NBS), reacted at room temperature for 20 hours. The solvent was removed to give a crude product of 1,3,5-tris(5'-bromo-2'-thienyl)benzene (3TB-3Br). Using petroleum ether as a flushing liquid, separate with a chromatographic column to obtain relatively pure 3TB-3Br, and then recrystallize to obtain pure 3TB-3Br.
[0038] In the second step, under the protection of nitrogen, 3,4-dioxyethylenethiophene (EDOT) and anhydrous tetrahydrofuran were added into a three-necked flask, and the reaction temperature was controlled at -78°C. After the temperature stabilized, n-butyllithium was slowly added dropwise, and after the dropwise addition, the temperature was raised to room temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com