Relugolix synthesis method
A purification method and compound technology, applied in the direction of organic chemistry, etc., can solve the problems of harsh reaction conditions and high equipment requirements, and achieve the effect of less side reactions, high purity and product purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
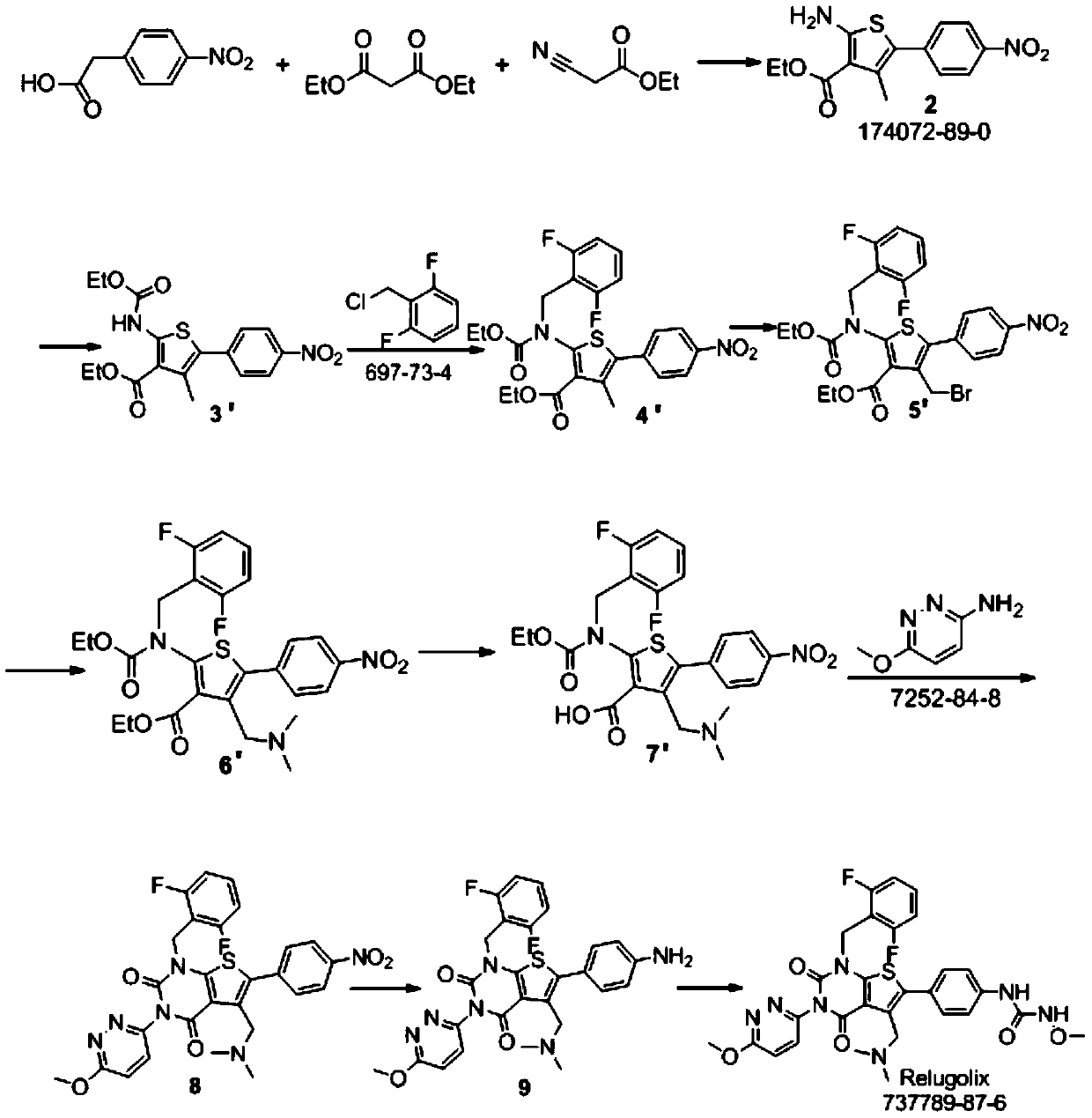
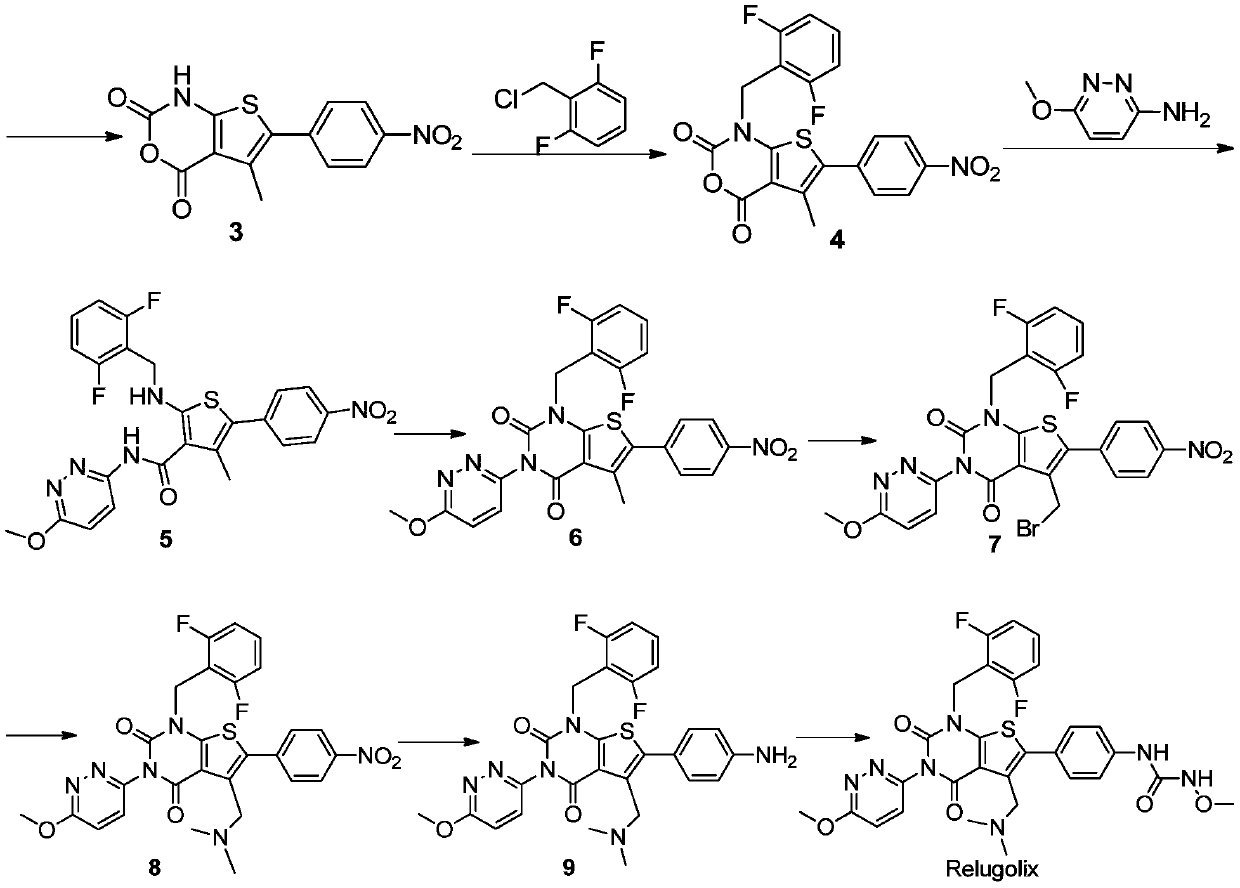
[0059] The synthesis of embodiment 1 Relugoli
[0060] According to the following synthetic route, the compound Relugoli was prepared:
[0061]
[0062]
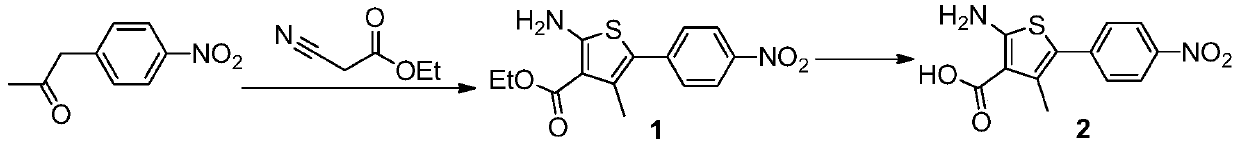
[0063] Synthesis of step 1, ethyl 2-amino-4-methyl-5-(4-nitrophenyl)-3-thiophenecarboxylate (compound 1):
[0064] 4-Nitropropiophenone (30g, 0.17mol), ethyl cyanoacetate (19.2g, 0.17mol) and ethanol (200ml) were added to the reaction flask, stirred and dissolved, then triethylamine (17.2g, 0.17mol ) and sulfur powder (5.4g, 0.17mol), heated to 50°C for 6h, and the reaction was complete by TLC monitoring. Concentrate to remove ethanol, add 300ml of ethyl acetate to the residue, extract with 100ml of saturated brine, and concentrate the organic phase to obtain a crude product. Then crystallize and purify with ethyl acetate / n-hexane (200ml / 200ml), filter, and dry to obtain ethyl 2-amino-4-methyl-5-(4-nitrophenyl)-3-thiophenecarboxylate (compound 1) 35.4g, yield 69%.
[0065] Synthesis of step 2, 2-amino-4-methyl-5-(4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com