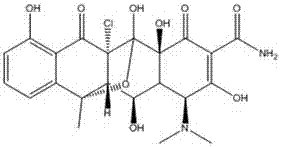
Preparation method of chlorinated terramycin
The technology of chloroxytetracycline and oxytetracycline is applied in the field of preparation technology of chloroxytetracycline, can solve the problems of large environmental harm, large toxic and side effects, low reaction yield and the like, and achieves low toxic and side effects, The effect of less waste residue and less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A preparation method of oxytetracycline, comprising the following steps:
[0020] 1) Add 500mL of methanol to a 1000mL reactor, control the temperature at 10-15°C, add 60g of oxytetracycline into the reactor and stir; after fully stirring for 1 hour, cool down to 5°C, add 30g of N-chloro Succinimide, stirred for 1h;
[0021] 2) Filter and separate the above materials, wash them with methanol, and discharge them after suction filtration for 30 minutes;
[0022] 3) The product obtained in step 2) was dried in a drying oven to finally obtain 58.83 g of oxytetracycline, an important intermediate of doxycycline, with a yield of 98%.
Embodiment 2
[0024] A preparation method of oxytetracycline, comprising the following steps:
[0025] 1) Add 5L of methanol to a 10L reactor, control the temperature at 10-15°C, add 0.6kg of oxytetracycline into the reactor and stir; after stirring for 1 hour, cool down to 0°C, and add 0.3kg of N - Chlorosuccinimide, stirred for 1h;
[0026] 2) Inject step 1) into the centrifuge for spinning, and wash with methanol, and discharge the material after spinning for 30 minutes;
[0027] 3) The product obtained in step 2) was dried in a blast drying oven to finally obtain 0.57 kg of oxytetracycline, an important intermediate of doxycycline, with a yield of 95%.
Embodiment 3
[0029] A preparation method of oxytetracycline, comprising the following steps:
[0030] 1) Add 500L of methanol to a 1000L reactor, control the temperature at 10-15°C, add 60kg of oxytetracycline into the reactor for stirring, stir thoroughly for 1 hour, cool down to 0°C and add 30kg of N-chloro Succinimide, stirred for 1h;
[0031] 2) Inject step 1) into the filter press for pressure filtration, and discharge after 1 hour of pressure filtration;
[0032] 3) The product obtained in step 2) was dried by an airflow drying system to finally obtain 59.33 kg of oxytetracycline, an important intermediate of doxycycline, with a yield of 98.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com