Preparation method of pharmaceutical intermediate 2-amine methylpyrazine hydrochloride
A kind of technology of aminomethylpyrazine hydrochloride and chloromethylpyrazine, which is applied in the field of preparation of 2-aminomethylpyrazine pharmaceutical intermediate 2-aminomethylpyrazine hydrochloride, and can solve harsh reaction conditions , 2-aminomethylpyrazine yield is low, the reaction process is not easy to control and other problems, to achieve the effect of cheap price and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
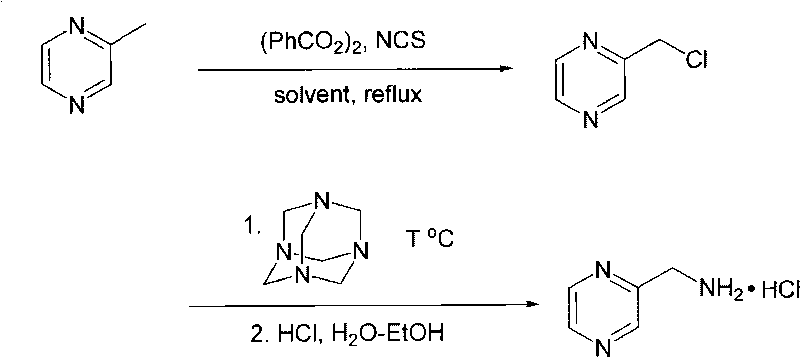
[0035] A kind of preparation method of pharmaceutical intermediate 2-aminomethylpyrazine hydrochloride of the present invention, the steps of its preparation method are as follows:
[0036] (1) Under the protection of nitrogen, 2-methylpyrazine, dibenzoyl peroxide and N-chlorosuccinimide with a molar ratio of 1:0.01~0.1:1~1.3 were added to the solvent anhydrous tetrachloride In carbonization, reflux reaction makes α-chloromethylpyrazine;
[0037] (2) After mixing the α-chloromethylpyrazine prepared above with catalyst potassium iodide and solvent anhydrous toluene, cool to below 15°C, and the molar ratio of α-chloromethylpyrazine to potassium iodide is 1: 0.01~0.1; Then add hexamethylenetetramine (hexamethylenetetramine) under stirring according to the molar ratio of α-chloromethylpyrazine and hexamethylenetetramine as 1: 1.0~1.3, control the temperature below 28°C, add After completion, the reaction temperature is 0-100°C, and the reaction time is 1-20 hours to obtain 2-chlo...
Embodiment 1
[0040] 1) Under nitrogen protection, add 2-methylpyrazine, dibenzoyl peroxide and N-chlorosuccinimide with a molar ratio of 1:0.01:1.2 into anhydrous carbon tetrachloride, and reflux The reaction makes α-chloromethylpyrazine;
[0041] 2) Mix the above-prepared α-chloromethylpyrazine with potassium iodide and anhydrous toluene and cool to below 15°C. The molar ratio of α-chloromethylpyrazine to potassium iodide is 1:0.1. Then, according to the molar ratio of α-chloromethylpyrazine and hexamethylenetetramine as 1:1, add hexamethylenetetramine (hexamethylenetetramine) under stirring, control the temperature below 28°C, and complete the addition Afterwards, the reaction temperature is 0° C., and the reaction time is 20 hours to obtain 2-chloromethylpyrazine hexamethylenetetramine double salt. Add concentrated hydrochloric acid and ethanol to the above reaction mixture, react at 30°C for 5 hours, cool, filter, wash, and dry to obtain the crude product of 2-aminomethylpyrazine hydr...
Embodiment 2
[0044] 1) Under nitrogen protection, add 2-methylpyrazine, dibenzoyl peroxide and N-chlorosuccinimide at a molar ratio of 1:0.1:1.0 to anhydrous carbon tetrachloride, and reflux The reaction makes α-chloromethylpyrazine;
[0045] 2) Mix the above-prepared α-chloromethylpyrazine with potassium iodide and anhydrous toluene and cool to below 15°C. The molar ratio of α-chloromethylpyrazine to potassium iodide is 1:0.05. Then add hexamethylenetetramine (hexamethylenetetramine) under stirring according to the molar ratio of α-chloromethylpyrazine and hexamethylenetetramine as 1:1.1, control the temperature below 28°C, after adding The temperature is 100° C. and the reaction time is 3 hours to obtain 2-chloromethylpyrazine hexamethylenetetramine double salt. Add concentrated hydrochloric acid and ethanol to the above reaction mixture, react at 10°C for 8 hours, cool, filter, wash, and dry to obtain the crude product of 2-aminomethylpyrazine hydrochloride.
[0046] 3) Recrystallize ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com