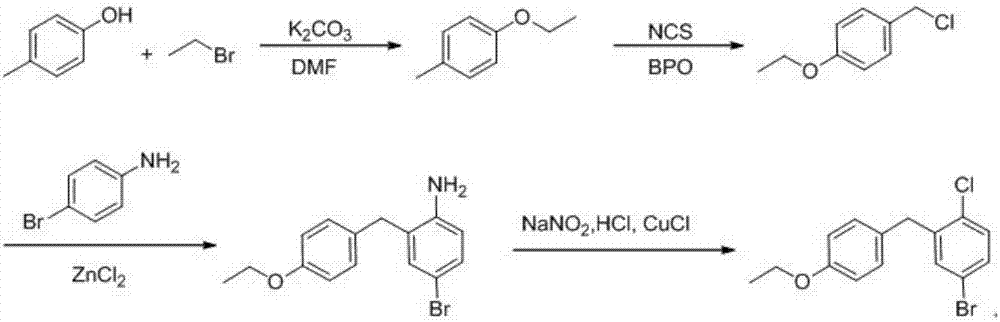
Preparation method of dapagliflozin intermediate
An intermediate and reaction technology, applied in the field of preparation of dapagliflozin intermediates, can solve the problems of highly toxic cyanide, serious pollution, etc., and achieve the effects of high product purity and short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The present invention will be further described below through the examples, but the examples do not limit the protection scope of the present invention.
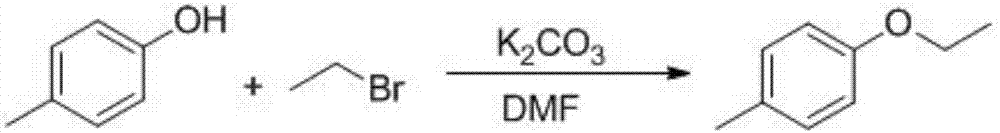
[0022] 1, Preparation of 4-ethoxytoluene
[0023] The reaction chemical formula is:
[0024]
[0025] Take 108kg of 4-methylphenol, dissolve in 200kgDMF, 50kg toluene, add potassium carbonate 150kg, at 60°, add dropwise the toluene solution of bromoethane (bromoethane 130kg, toluene 150kg), dropwise in 30 minutes, add dropwise Completed, reacted at 60 ° for 2 hours, after the reaction, filtered to remove potassium bromide and potassium carbonate, recovered the solvent under reduced pressure, added 400kg of water to the residual liquid, extracted with 400kg of ethyl acetate, dried over anhydrous sodium sulfate, filtered, recovered the solvent from the filtrate, and left The product was distilled under reduced pressure to obtain 127.8 kg of a light yellow liquid with a yield of 94%.
[0026] 2, Preparation of 4-eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com