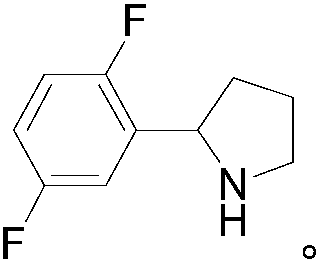
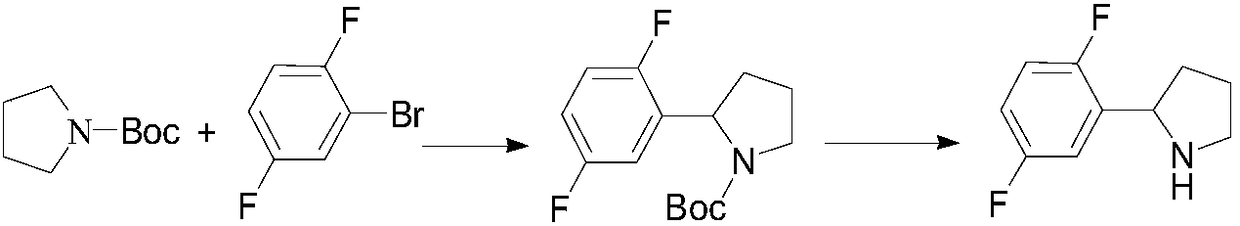
Preparation method of 2-(2,5-difluorophenyl) pyrrolidine
A technology of difluorophenyl and pyrrolidine, which is applied in the field of preparation of 2-pyrrolidine, can solve the problems of unfavorable industrialization, violent reaction, and low reaction temperature, and achieve reasonable synthesis process design, mild reaction conditions, and low raw material cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] An embodiment of the preparation method of 2-(2,5-difluorophenyl)pyrrolidine described in the present invention, the preparation method of 2-(2,5-difluorophenyl)pyrrolidine described in this embodiment comprises The following steps:
[0039] (1), the preparation of 1-chloropyrrolidine: in the reaction flask equipped with thermometer, under nitrogen protection, add N-chlorosuccinimide (43.37g, 325mmol, 1.1eq), methyl tert-butyl ether 160mL, stirred to lower the temperature below 20°C, slowly added pyrrolidine (21.00g, 295mmol, 1.0eq) dropwise, stirred at room temperature for 24 hours, and stopped the reaction; Wash with 80 mL of 20% brine, dry over anhydrous magnesium sulfate, and distill off the solvent under reduced pressure at a temperature not higher than 25°C to obtain 31.00 g of light yellow oil, namely 1-chloropyrrolidine, with a yield of 99%;
[0040] (2), the preparation of 3,4-dihydro-2H-pyrrole: in the reaction bottle equipped with thermometer, nitrogen prote...
Embodiment 2
[0044]An embodiment of the preparation method of 2-(2,5-difluorophenyl)pyrrolidine described in the present invention, the preparation method of 2-(2,5-difluorophenyl)pyrrolidine described in this embodiment comprises The following steps:
[0045] (1), the preparation of 1-chloropyrrolidine: with embodiment 1;
[0046] (2), preparation of 3,4-dihydro-2H-pyrrole: same as Example 1;
[0047] (3), the preparation of 2-(2,5-difluorophenyl) pyrrolidine: in the reaction flask that reflux tube is housed, constant pressure drop filter funnel and thermometer, nitrogen protection, add magnesium chip (2.54g , 104mmol, 1.2eq), 20mL of anhydrous tetrahydrofuran, 1-bromoethane (0.2mL), dropwise added 2,5-difluorobromobenzene (16.80g, 87mmol, 1.0eq, dissolved in 35mL of anhydrous THF) about 1 / 4, stir to raise the temperature to 45°C, stir for 0.5 hours, start the reaction, continue to add 2,5-difluorobromobenzene solution dropwise slowly, keep the reaction for 2 hours after the addition, c...
Embodiment 3
[0049] An embodiment of the preparation method of 2-(2,5-difluorophenyl)pyrrolidine described in the present invention, the preparation method of 2-(2,5-difluorophenyl)pyrrolidine described in this embodiment comprises The following steps:
[0050] (1), the preparation of 1-chloropyrrolidine: with embodiment 1;
[0051] (2), preparation of 3,4-dihydro-2H-pyrrole: same as Example 1;
[0052] (3), the preparation of 2-(2,5-difluorophenyl) pyrrolidine: in the reaction flask that reflux tube is housed, constant pressure drop filter funnel and thermometer, nitrogen protection, add magnesium chip (3.80g , 156mmol, 1.8eq), anhydrous tetrahydrofuran 20mL, 1-bromoethane (0.2mL), dropwise added 2,5-difluorobromobenzene (25.10g, 130mmol, 1.5eq, dissolved in 50mL anhydrous THF) about Quarter, stir and heat up to 45°C, stir for 0.5 hours, start the reaction, continue to slowly add 2,5-difluorobromobenzene solution dropwise, keep warm for 2 hours after the addition, cool down to 25°C, dro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com