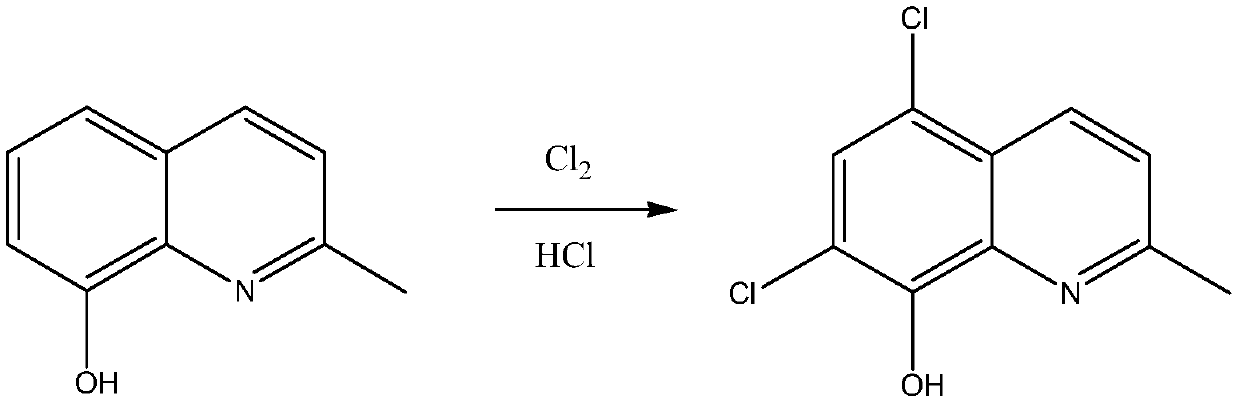
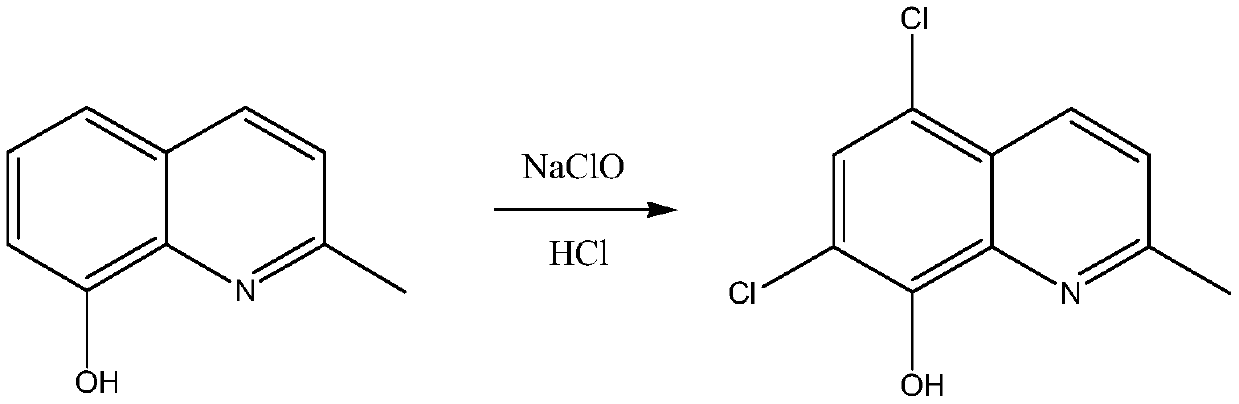
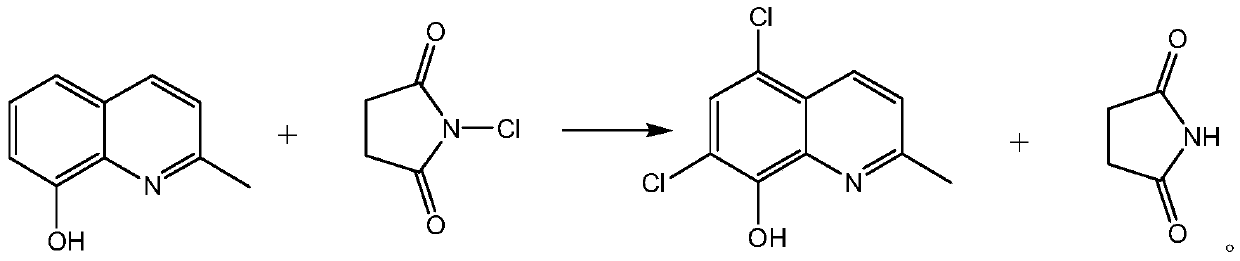
Synthetic technology of chlorquinaldol
A technology of chloroquinadol and synthesis process, applied in the field of drug synthesis, can solve the problems of large amount of sodium hypochlorite solution, increased cost of treating waste liquid, large water content, etc., and achieves increased conversion rate and yield, reduced operation, and reduced waste. effect of liquid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A kind of synthetic technique of chloroquinaldol: under the dark condition, in the four-neck bottle of 250 milliliters, drop into 100 grams of dichloromethane and 10 grams of 8-hydroxyl-2-methylquinoline, cool to-5 ℃, add 16.8 g of N-chlorosuccinimide and 0.08 g of aluminum chloride were incubated at 0±5°C for 12 hours, filtered with suction, and the solvent was concentrated to obtain crude chloroquinaldol. Refined with 135 grams of ethanol and 45 grams of purified water to obtain chloroquinaldol with a yield of 98.6% and an HPLC purity of 99.93%.
Embodiment 2
[0029] A kind of synthetic technique of chloroquinaldol: under dark condition, in the four-neck bottle of 500 milliliters, drop into 300 grams of chloroform and 20 grams of 8-hydroxyl-2-methylquinoline, cool to 10 ℃, add 34.42 grams of N - chlorosuccinimide and 0.34 g of aluminum chloride, heat at 15±5° C. for 10 hours, filter with suction, and concentrate the solvent to obtain crude chloroquinaldol. Refined with 266 grams of ethanol and 14 grams of purified water to obtain chloroquinaldol with a yield of 98.2% and an HPLC purity of 99.91%.
Embodiment 3
[0031] A kind of synthetic technique of chloroquinaldol: under dark condition, in the four-neck bottle of 500 milliliters, drop into 200 grams of chloroform and 20 grams of 8-hydroxyl-2-methylquinoline, add 32.75 grams of N-chlorobutane Imide and 0.51 g of aluminum chloride were heated up to 55±5°C and kept for 6 hours, filtered with suction, and the solvent was concentrated to obtain crude chloroquinaldol. Refined with 238 grams of ethanol and 42 grams of purified water to obtain chloroquinaldol with a yield of 98.4% and an HPLC purity of 99.96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com