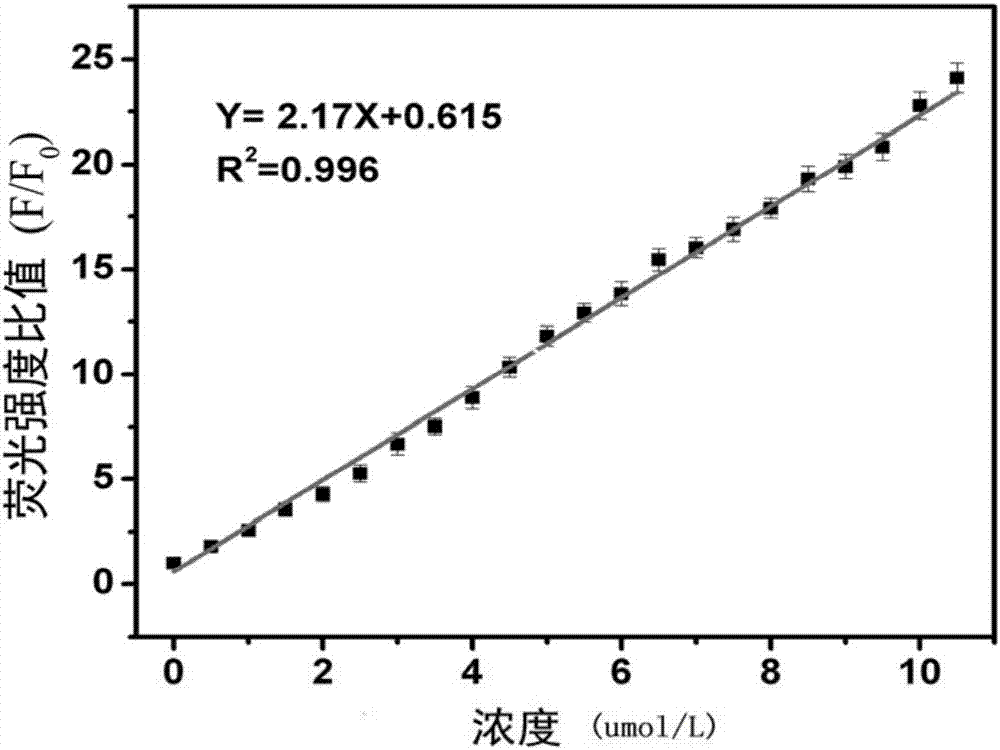
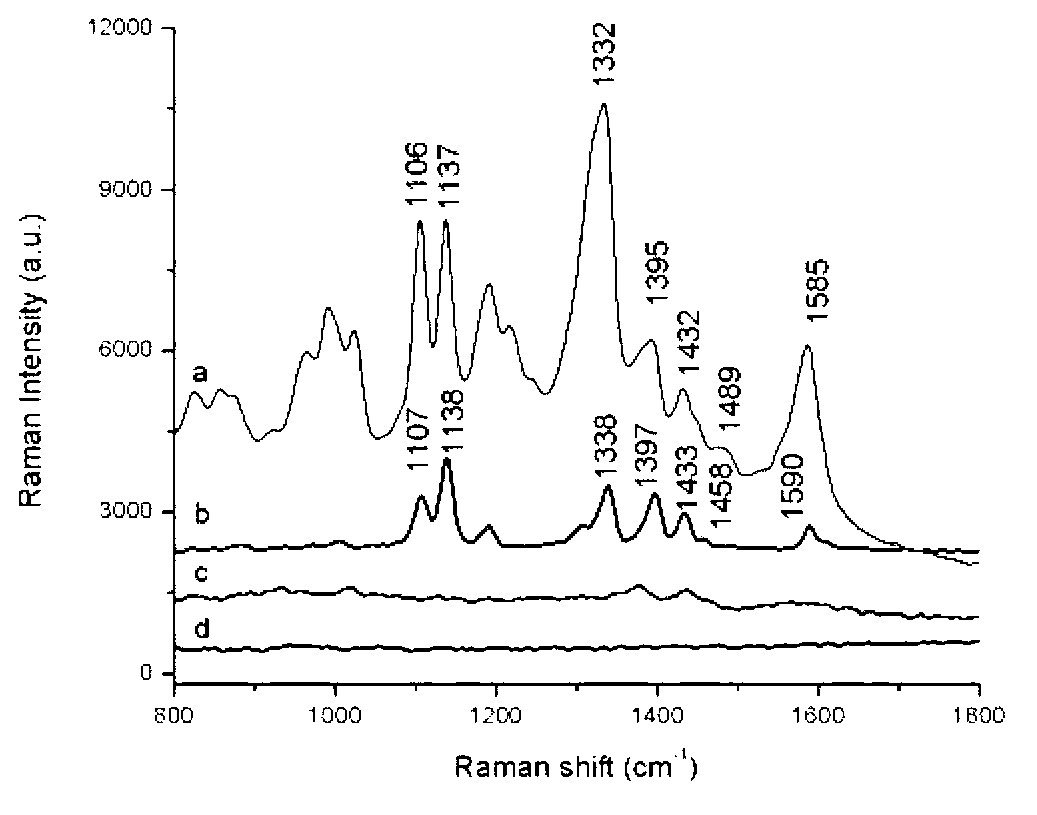
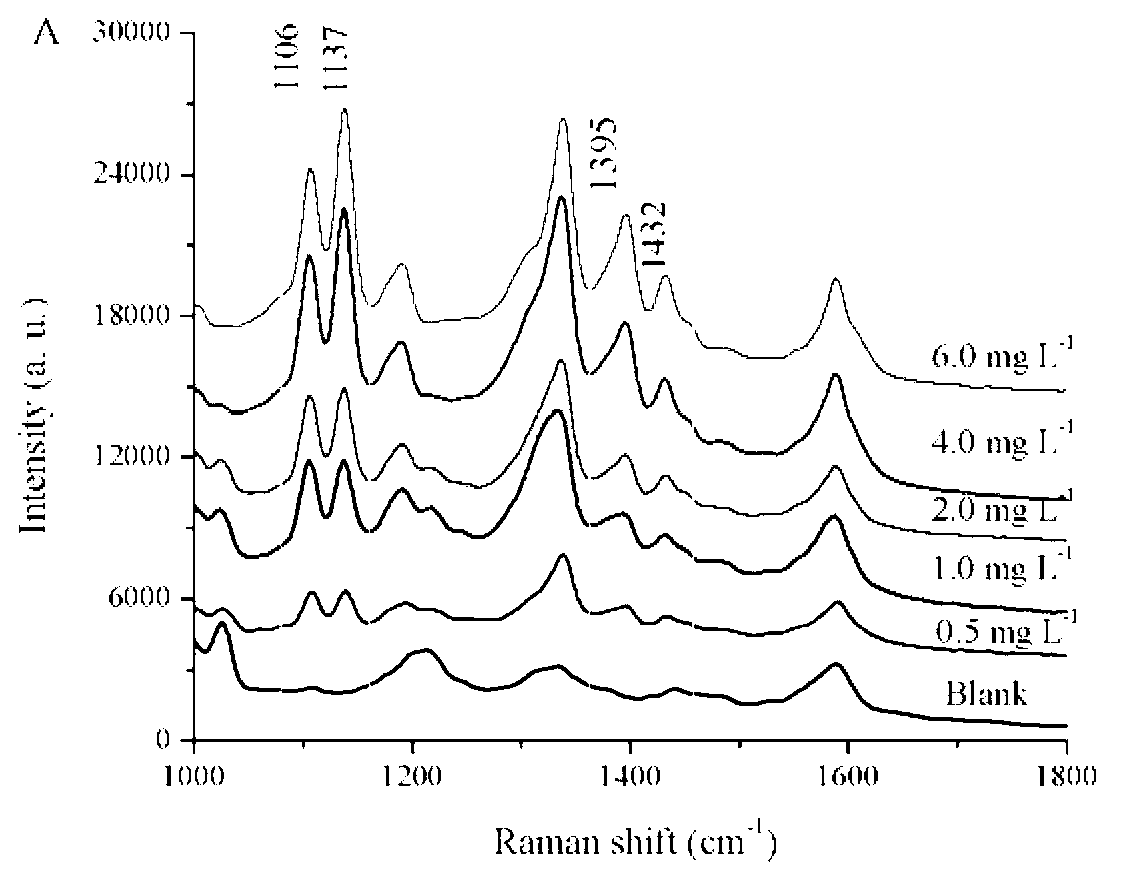
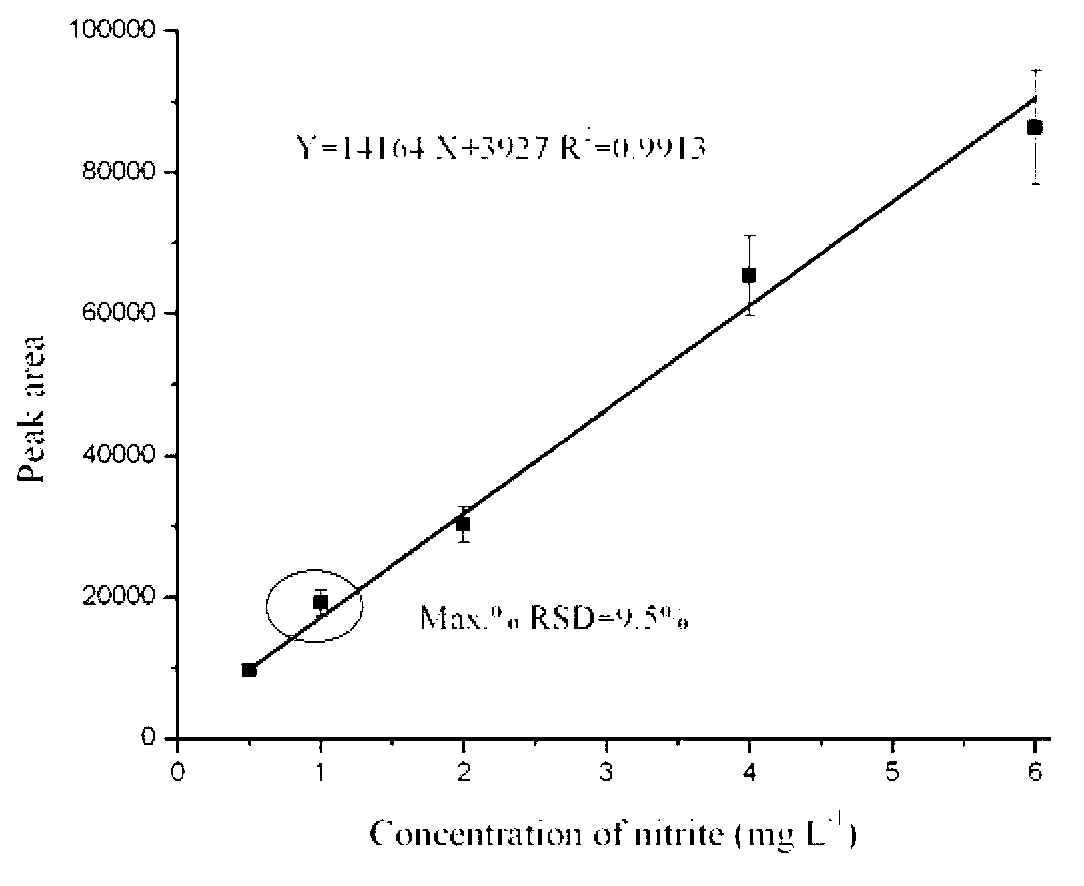
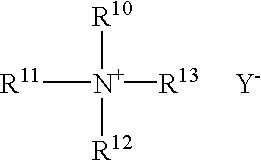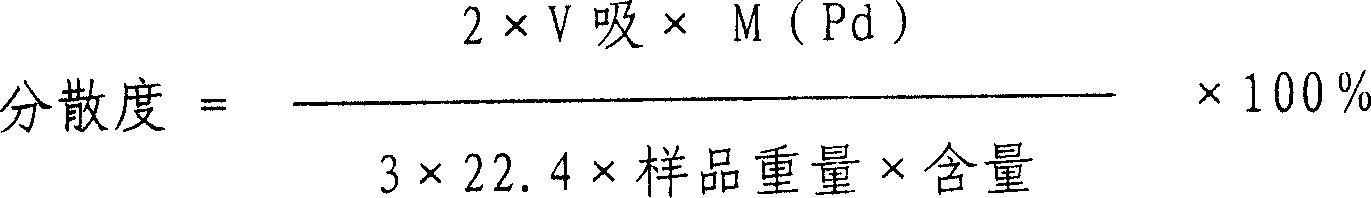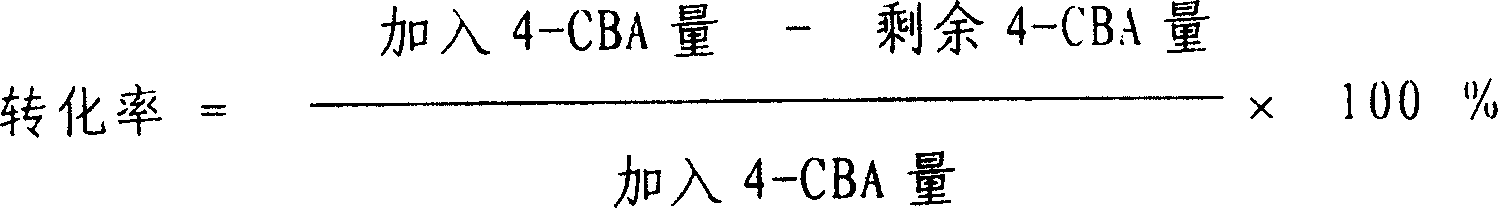Patents
Literature
238 results about "Nitrite ion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
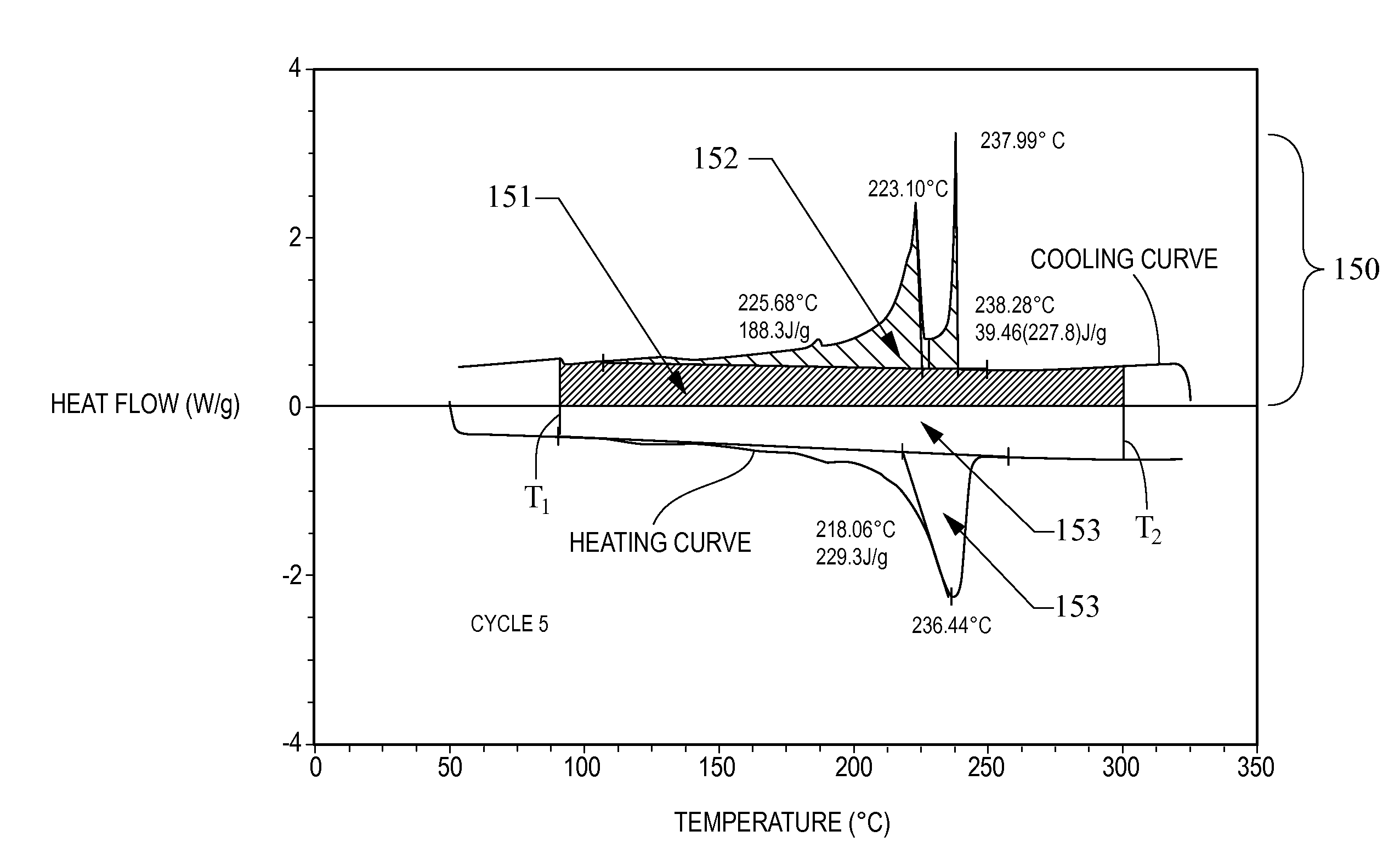
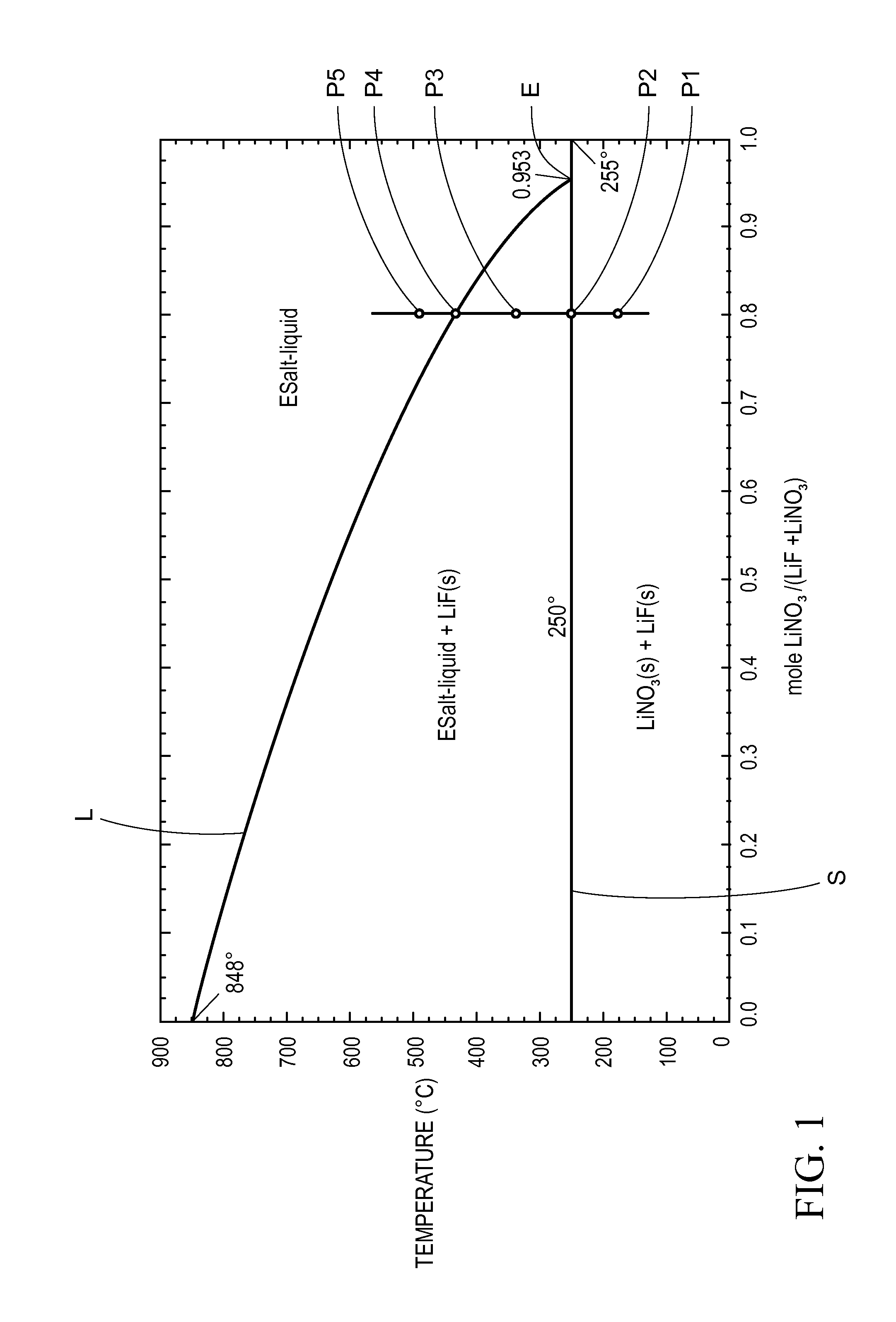
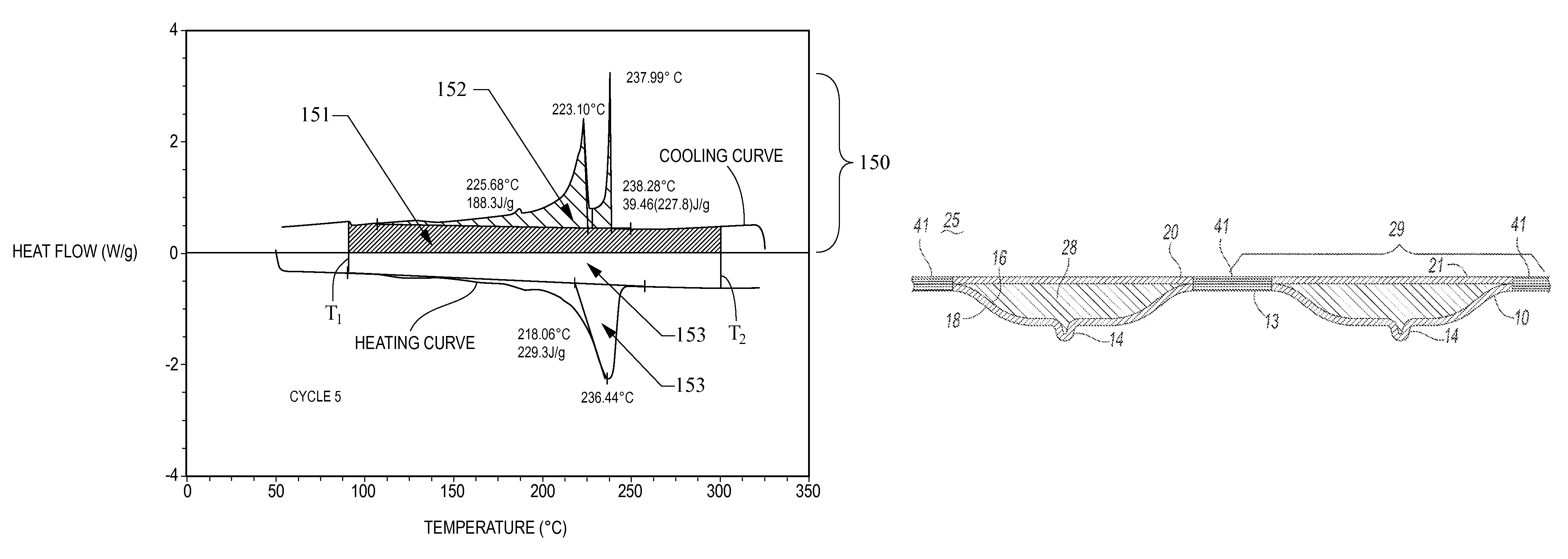
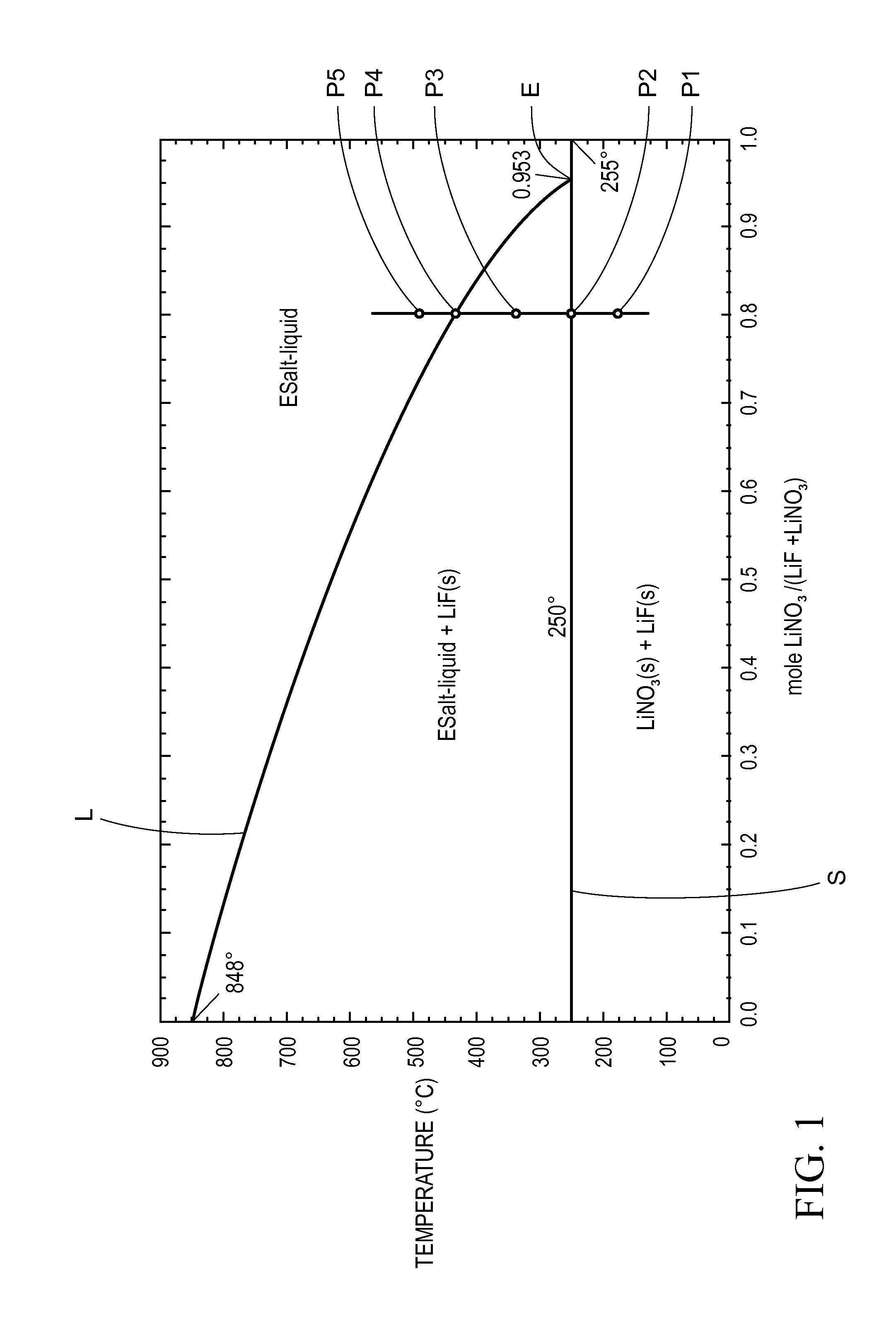
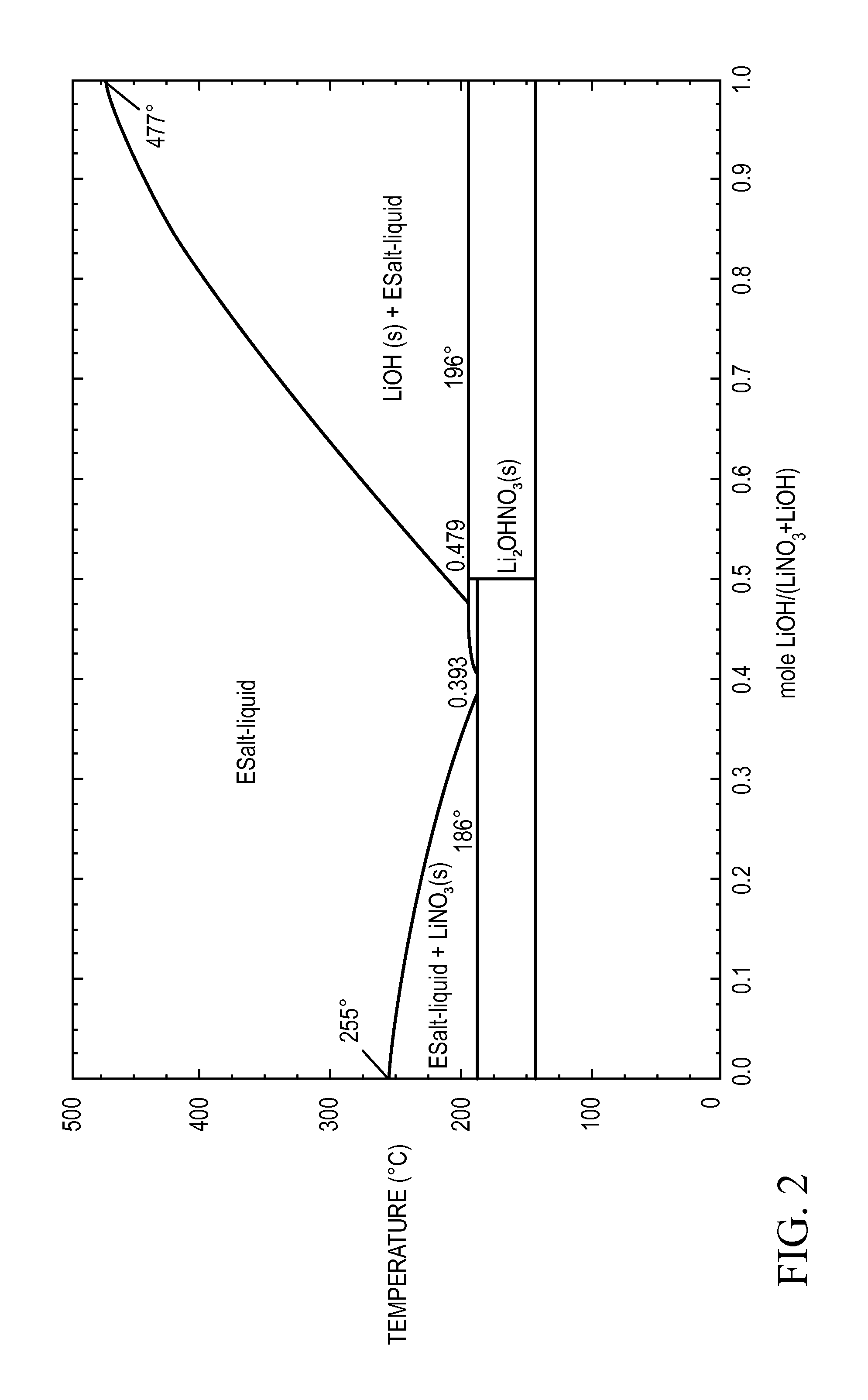
Thermal energy storage materials
A thermal energy storage material (TESM) system (and associated methods) that reproducibly stores and recovers latent heat comprising i) at least one first metal containing material including at least one first metal compound that includes a nitrate ion, a nitrite ion, or both; ii) at least one second metal containing material including at least one second metal compound; and iii) optionally including water, wherein the water concentration if any is present is less than about 10 wt. %; wherein the TESM has a liquidus temperature, TL, from about 100° C. to about 250° C.; and wherein the TESM exhibits a heat storage density from 300° C. to 80° C. of at least about 1 MJ / l; so that upon being used in a system that generates heat, at least a portion of the heat is captured and stored by the TESM and subsequently released for use, and the system is generally resistant to corrosion at temperatures of about 300° C.
Owner:DOW GLOBAL TECH LLC
Transdermal pharmaceutical delivery compositions
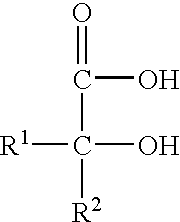
A pharmaceutically delivery system and method of use therefor are described comprising a pharmaceutically active agent and acidified nitrite as an agent to produce local production of nitric oxide at the skin surface. The dosage form may be in any pharmaceutically acceptable carrier means and comprises an acidifying agent adapted to reduce the pH at the environment. In one embodiment, a barrier consisting of a membrane allows diffusions of the anaesthetic and nitrite ions while preventing direct contact of the skin and acidifying agent.
Owner:QUEEN MARY UNIV OF LONDON
Reducing sulfide in production fluids during oil recovery
Methods are provided for treating production fluid in a production well in an oil reservoir to reduce the amount of sulfide in the production fluid. The production fluid is treated with nitrate and / or nitrite ions or inorganic oxidizing agent in an aqueous solution that is added to the well casing.
Owner:EI DU PONT DE NEMOURS & CO
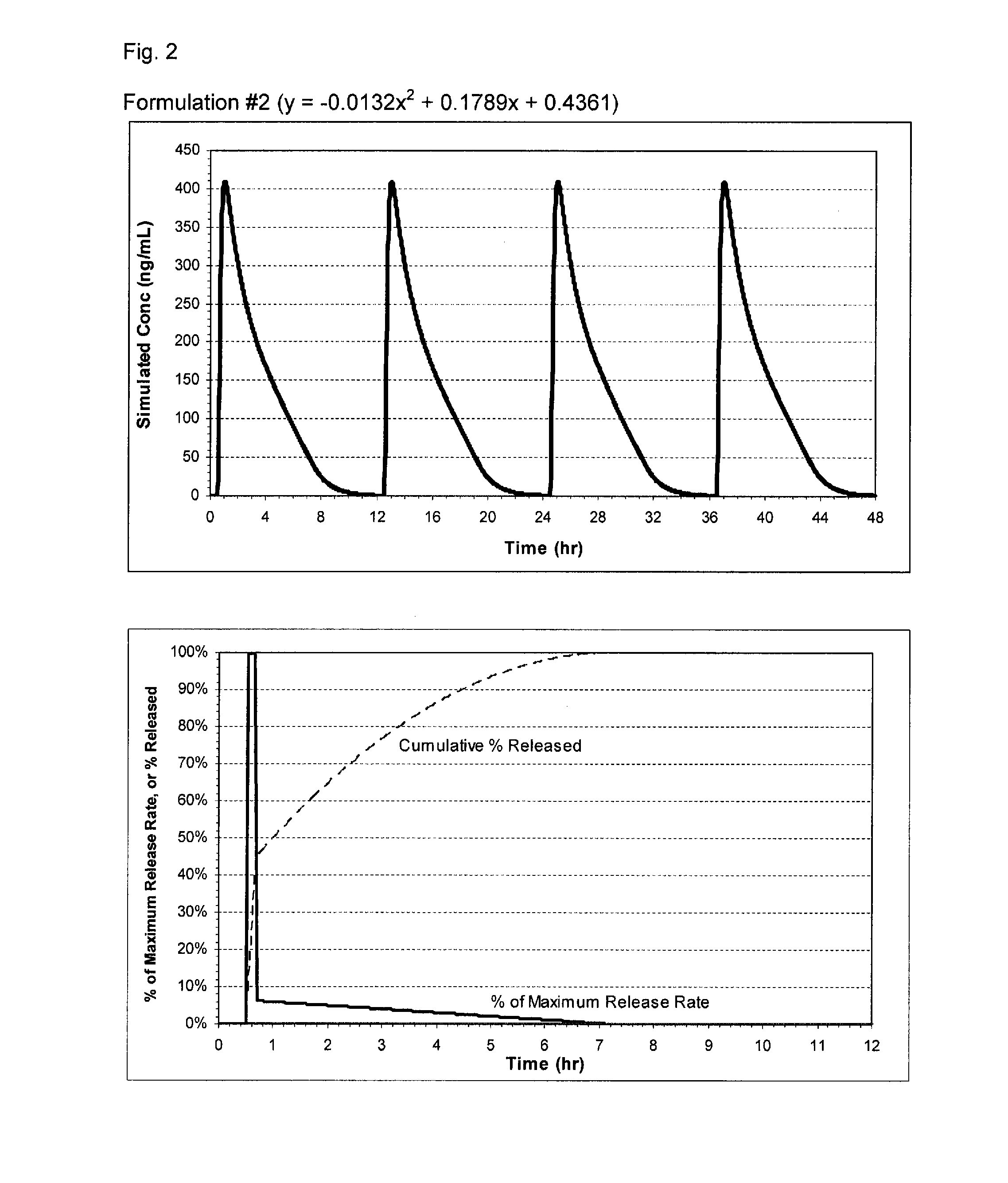
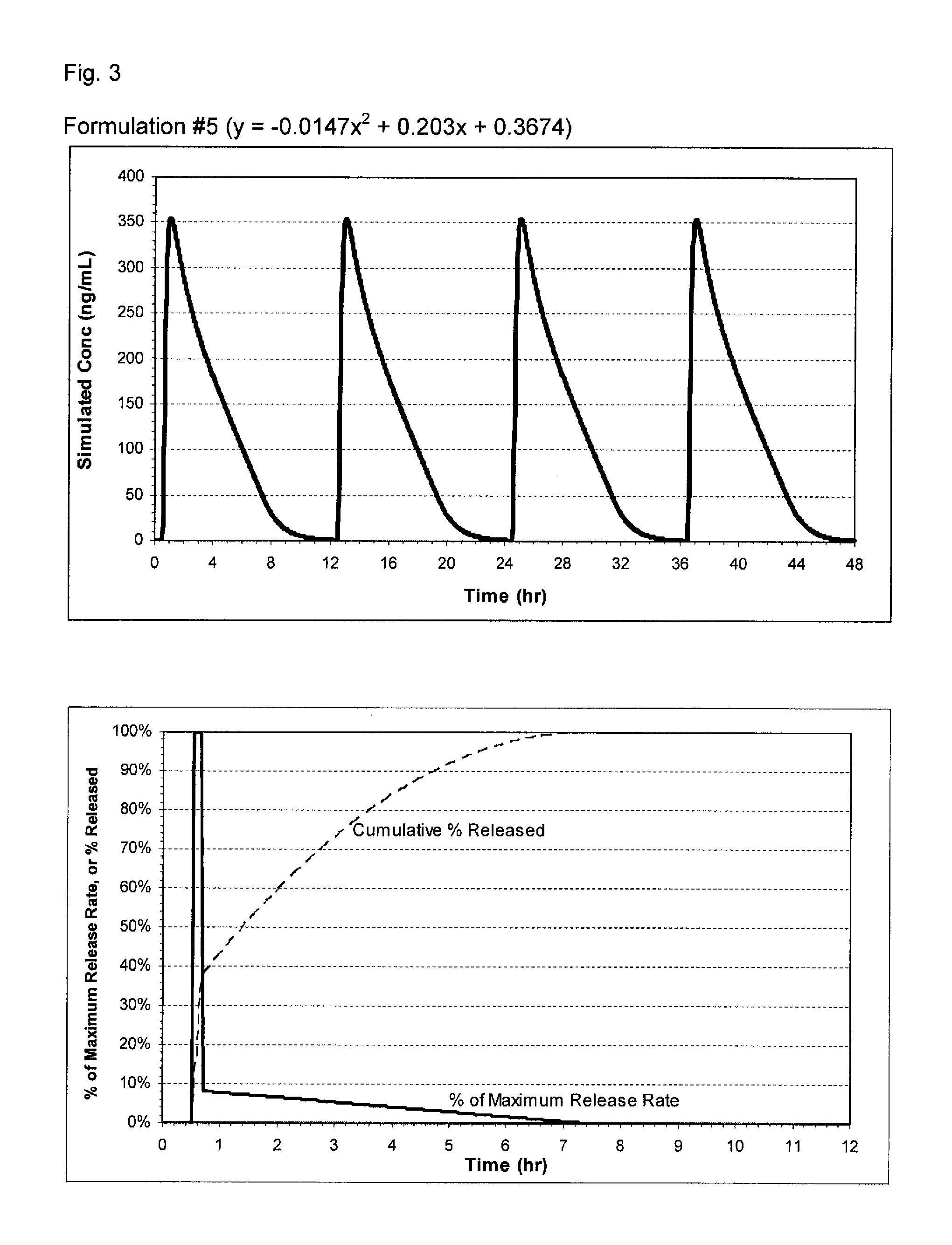
Pharmaceutical formulations of nitrite and uses thereof
The present invention relates to pharmaceutical compositions of nitrites such as inorganic nitrites, or any pharmaceutically acceptable salts, solvates, or prodrugs thereof, and the medical use of these compositions. The pharmaceutical compositions, which can be formulated for oral administration, can provide immediate release or extended release of the nitrite ion (NO2−). The pharmaceutical compositions of the invention are useful, for example, for the treatment of chronic tissue ischemia.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Disinfecting nitrous acid compositions and process for using the same
InactiveUS20030175362A1Significant antimicrobial activityMinimum corrosionHeavy metal active ingredientsBiocideDiseaseNitrite ion
The present invention relates generally to compositions and methods for the use of nitrous acid solutions to disinfect inanimate surfaces and animal tissues, and to treat diseases and wounds. More specifically, the invention deals with the partial and selected conversion of nitrite ion to nitrous acid in order to optimize the germicidal efficacy and duration of the nitrous acid consistent with the nature of the intended application.
Owner:KROSS ROBERT D +1
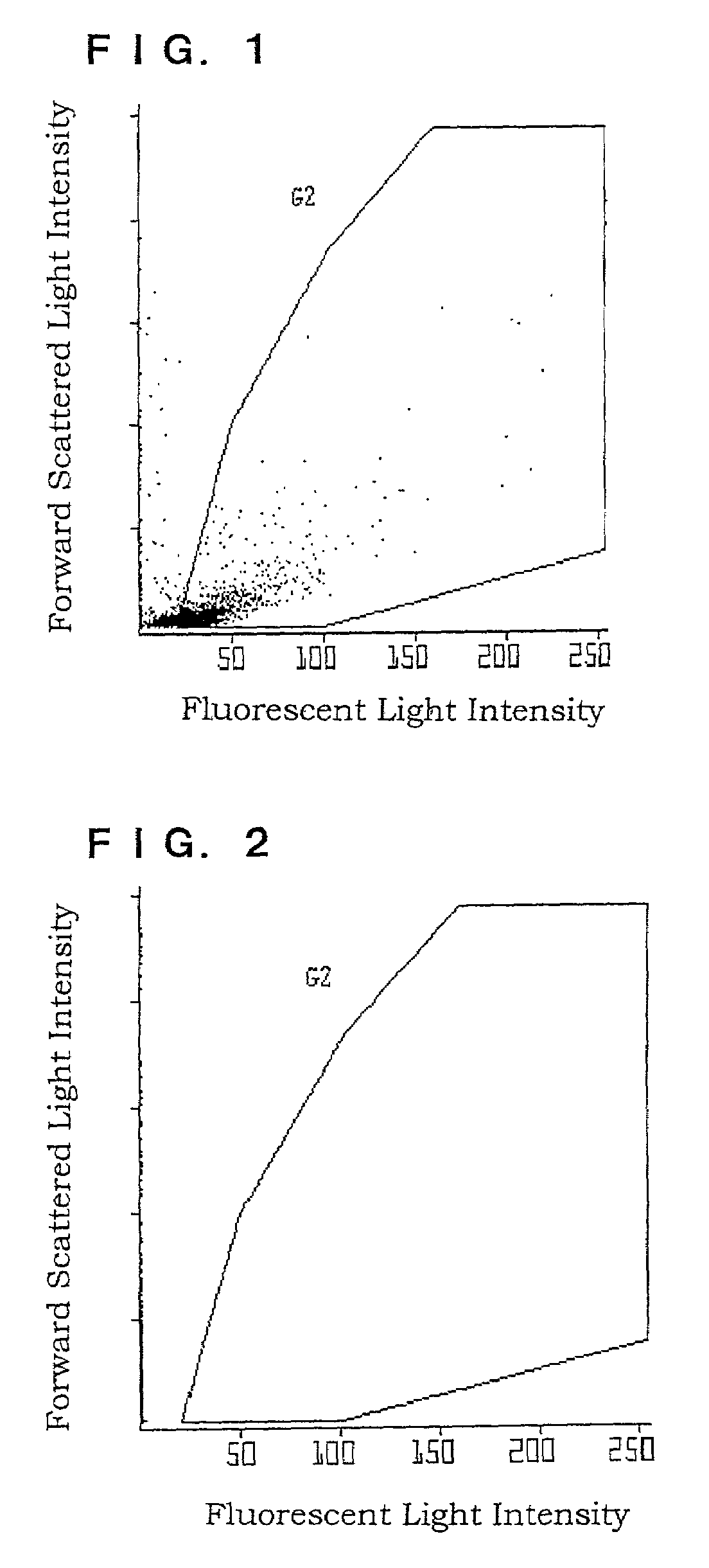
Method of staining, detection and counting bacteria, and a diluent for bacterial stain
InactiveUS7309581B2Efficient and fast detectionMicrobiological testing/measurementPreparing sample for investigationNitrite ionPolymethine dye
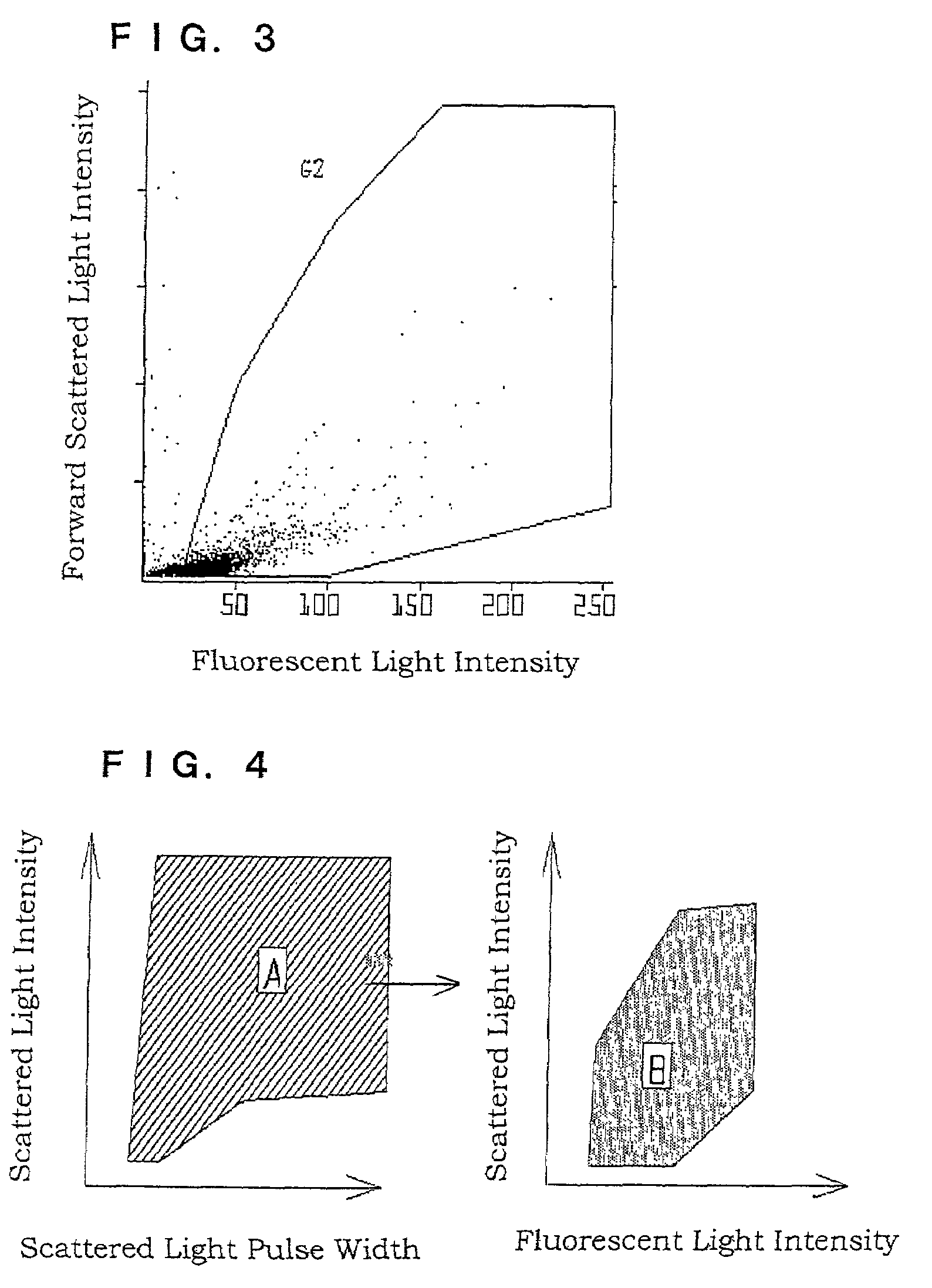
A method of staining bacteria comprises: working a polymethine dye on a sample in the presence of a substance capable of reducing nitrite ions to stain bacteria in the sample. A method of detecting bacteria comprises the following steps of: (1) working a polymethine dye on a sample by a method as described above to stain bacteria in the sample, (2) introducing the thus treated sample into a detecting part of a flow cytometer and irradiating cells of the stained bacteria one by one with light to measure scattered light and fluorescent light emitted from each of the cells; and (3) discriminating the bacteria from other components in accordance with an intensity of a scattered light signal and an intensity of a fluorescent light signal or a pulse width reflecting the length of particles to count the bacteria.
Owner:SYSMEX CORP
Transdermal pharmaceutical delivery compositions
A pharmaceutically delivery system and method of use therefor are described comprising a pharmaceutically active agent and acidified nitrite as an agent to produce local production of nitric oxide at the skin surface. The dosage form may be in any pharmaceutically acceptable carrier means and comprises an acidifying agent adapted to reduce the pH at the environment. In one embodiment, a barrier consisting of a membrane allows diffusions of the anaesthetic and nitrite ions while preventing direct contact of the skin and acidifying agent.
Owner:QUEEN MARY UNIV OF LONDON
Method for eliminating oxynitride from air flow and the special equipment thereof
InactiveCN101032678ALow costInexpensive to operateDispersed particle separationNitrite ionElectrolysis
The method of eliminating nitrogen oxide from gas flow is to lead the gas flow into an absorption tower, where the NO2 in the gas flow is absorbed directly by the absorbing liquid, the NO is oxidized by the chlorine the electrically catalytic oxidizing reactor into NO2 absorbed by the absorbing liquid and nitrite ion in the absorbed product is further oxidized into chemically stable nitrate ion, so as to reach gas purification and avoid secondary pollution. The gaseous oxidant and liquid oxidant are regenerated through an electrolysis process, and the absorbed liquid is returned with the circulating pump to the absorbing tower for reuse. The method has high absorption efficiency, no need of throwing oxidant and greatly lowered running cost. The present invention also discloses corresponding apparatus.
Owner:黄立维
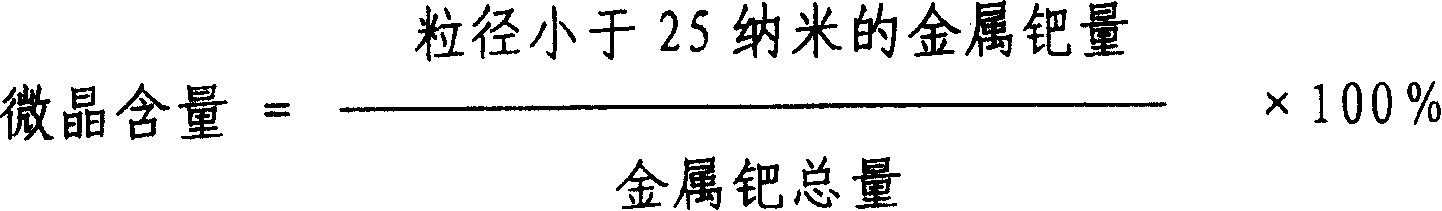
Method for preparing palladium/carbon catalyzer by hydrogenising and refining crude terephthalic acid
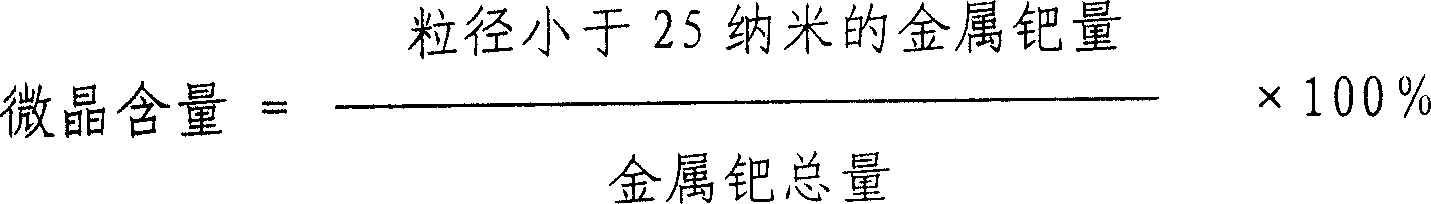
InactiveCN1565726AGood dispersionHigh microcrystalline contentOrganic compound preparationCarboxylic compound preparationNitrite ionPhosphoric acid
A method for preparing Pd / C catalyst for selective hydrogenation is provided. The content of Pd in catalyst is 0.2 to 5% by weight. The preparing process includes the following steps: active carbon carrier is washed by 0.1 to 5N concentration of acid washing liquid selected from hydrochloric acid, nitric acid or phosphoric acid; active carbon carrier is washed to neutrality by water and dried; the active carbon carrier is soaked in nitrite ion containing solution for 2 to 24 hours and dried; the Pd compound is sprayed on the active carbon carrier to form catalyst precursor, after aging and reduction process, the catalyst product is obtained. Metal Pd in catalyst has higher dispersion degree and the content of crystallite is very high.
Owner:CHINA PETROLEUM & CHEM CORP
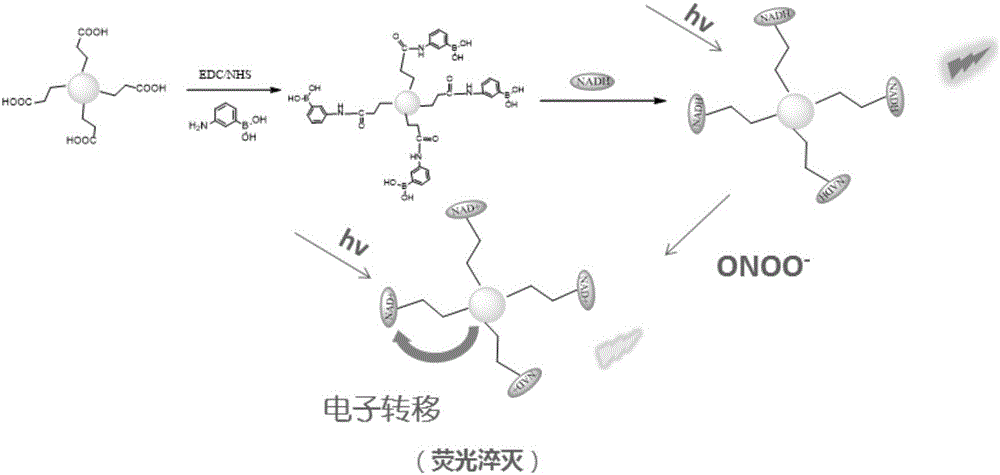
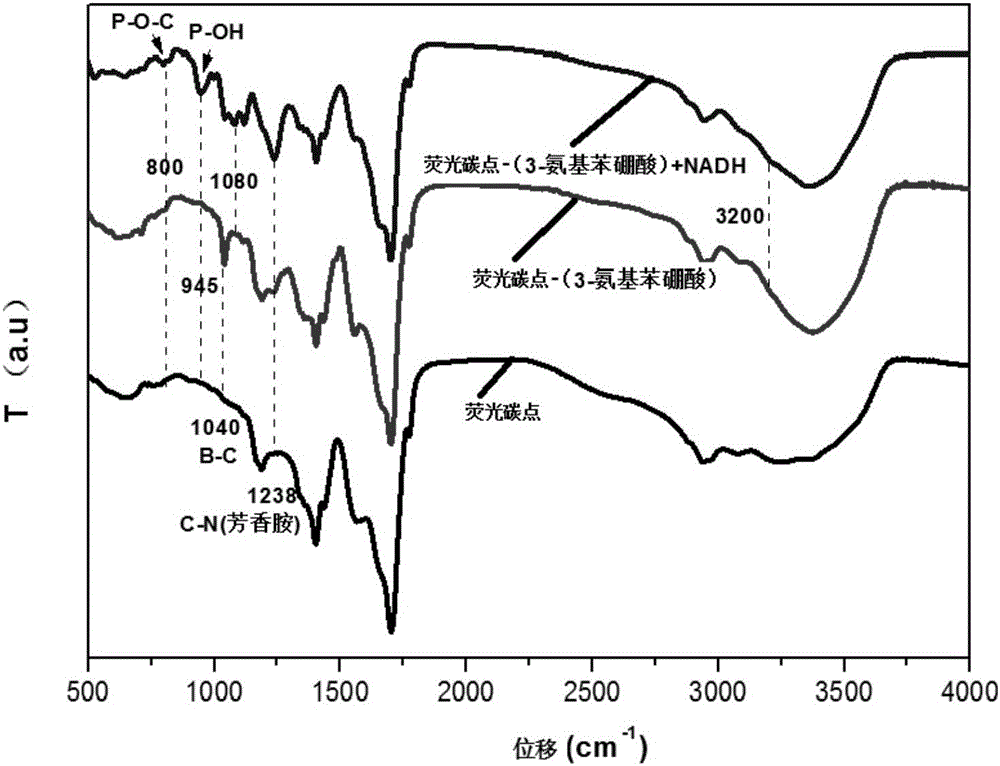
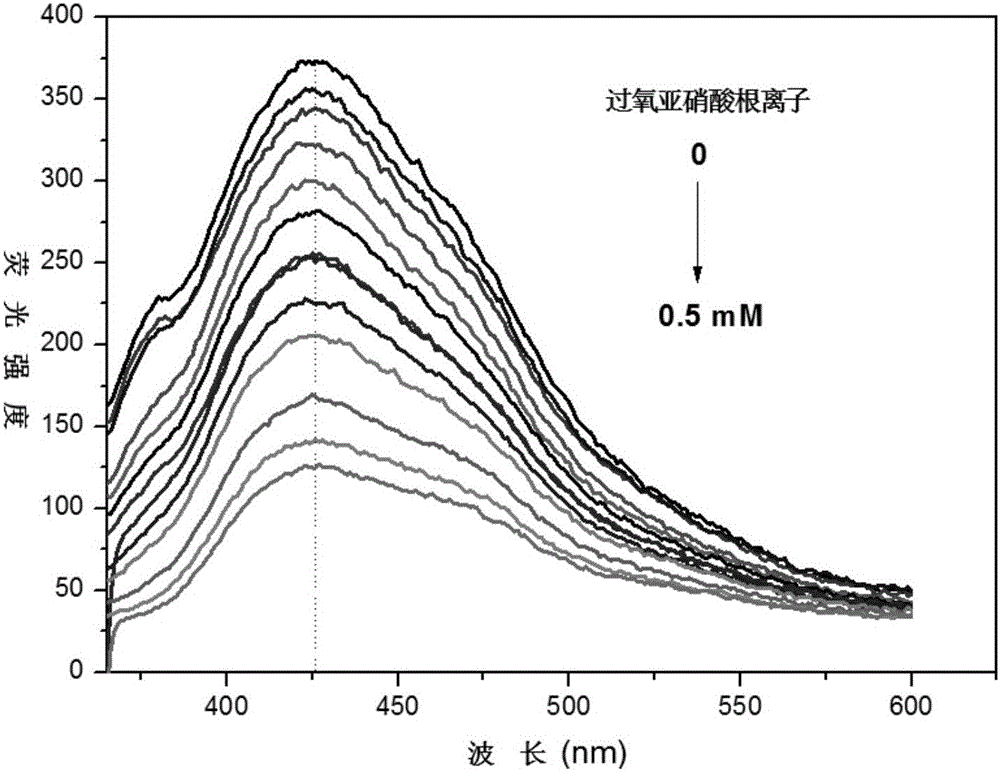
Fluorescent probe for detecting peroxynitrite in cell and synthetic method thereof
ActiveCN107286927AGood choiceDetection has no effectGroup 3/13 element organic compoundsFluorescence/phosphorescenceHigh cellNitrite ion
The invention provides a fluorescent probe for detecting peroxynitrite in a cell. The structural formula of the fluorescent probe is shown in the description, wherein R1 and R2 are selected from hydrogen, linear chain alkyl containing 1 to 5 carbon atoms or naphthenic base containing 3 to 8 carbon atoms respectively and separately; R3 is selected from a cyano group or an ester group. The fluorescent probe has high peroxynitrite selectivity, high cell permeability, extremely-short response time, extremely high detection sensitivity and no toxic and side effects to the cell per se, and is suitable for detecting the concentration change of the peroxynitrite in the cell. The fluorescent probe synthetic method is simple, is high in yield, and is suitable for large-scale production.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Method for rapidly detecting nitrite by using surface enhanced Raman spectrum and application thereof
The invention relates to a method for rapidly detecting nitrite by using a surface enhanced Raman spectrum, and the application of the method. A Raman signal of the nitrite self is very weak, an SERS (surface enhanced Raman spectrum) signal is also weak, a coupling dye is generated by using nitrite ions and a diazonium-coupling reagent under an acid or weak alkaline condition, the SERS signal of the coupling dye is strong, and the qualitative and quantitative detection is carried out on the nitrite by indirectly measuring the amount of the coupling dye through the SERS. According to the method, Au / SiO2 is used as an SERS substrate material, and the stability of particles is improved by a silica shell layer, so that the result repeatability is good. The method is applicable to rapid detection of the nitrite in complex samples such as a food sample, an environment sample and a biological sample, and has the advantages of simplicity, rapidness (about five minutes), high selectivity, on-site detection applicability, convenience in popularization and use and the like.
Owner:SUN YAT SEN UNIV
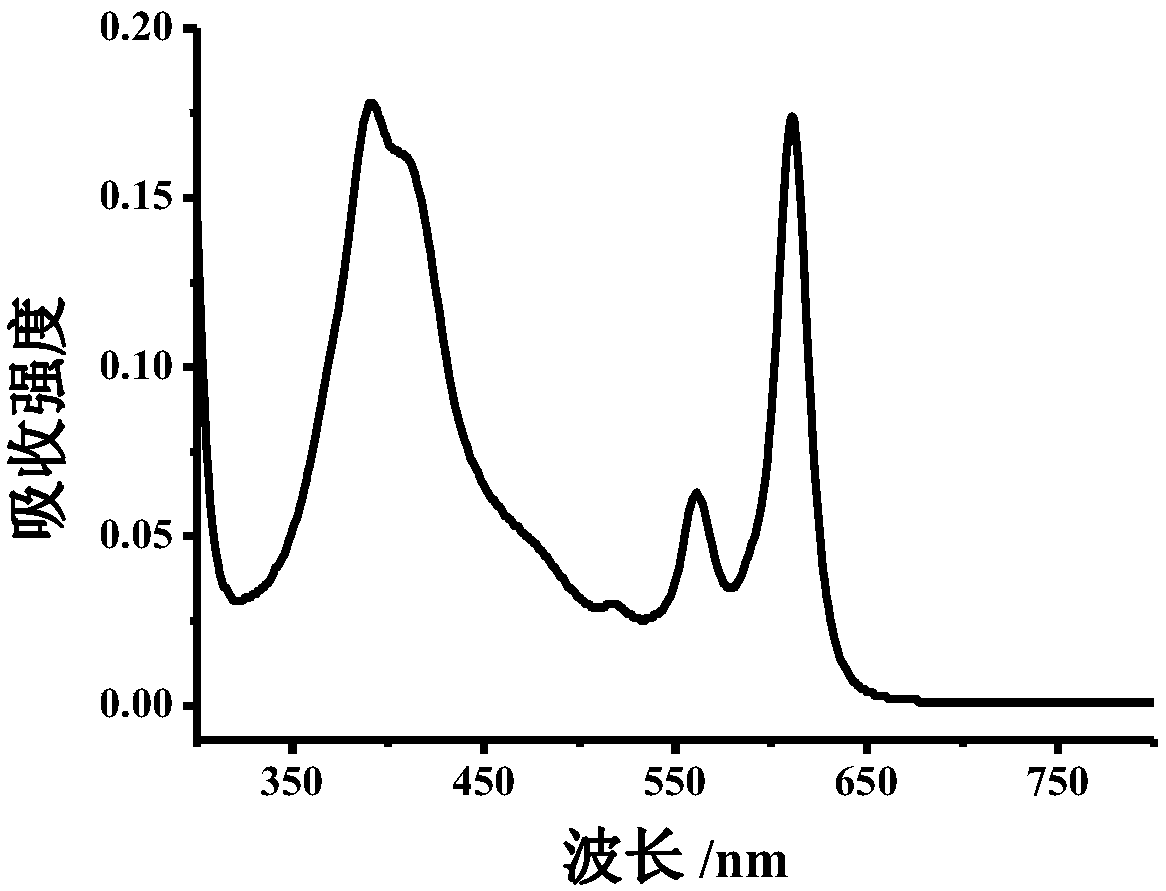
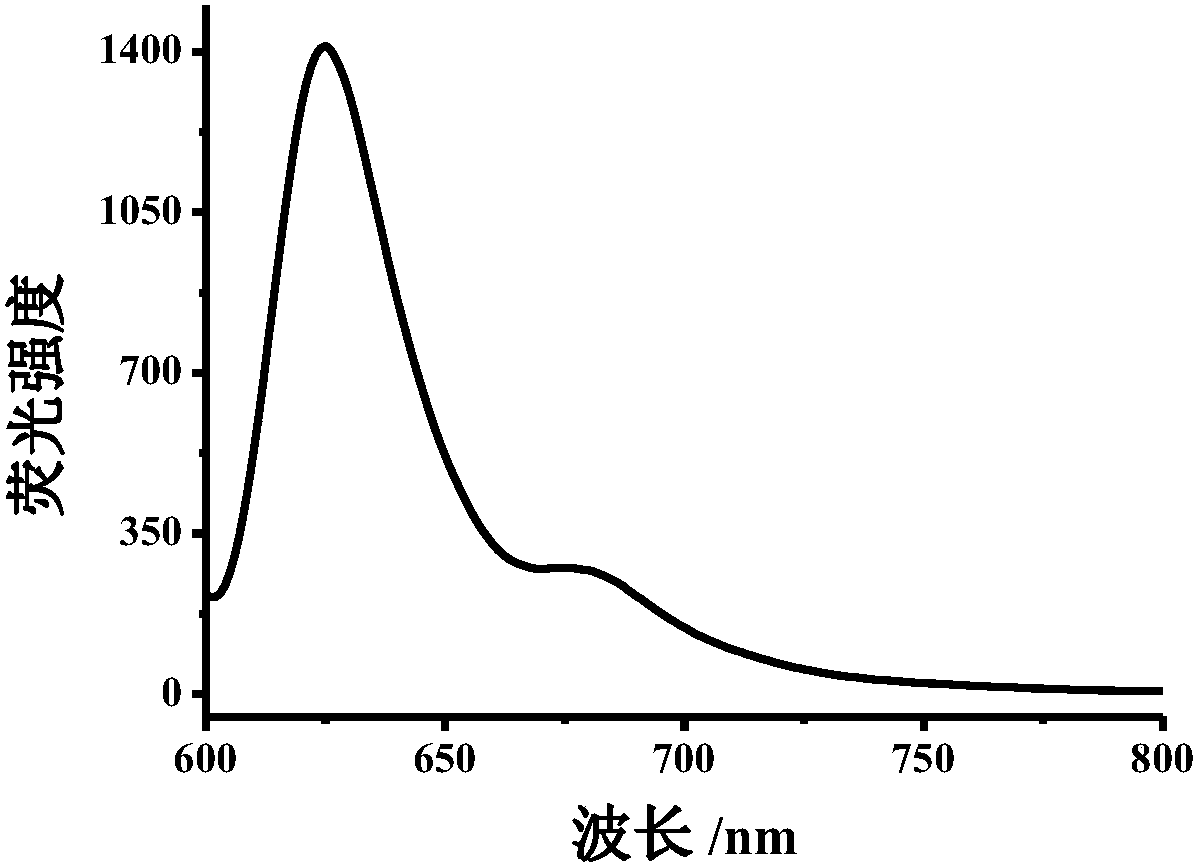
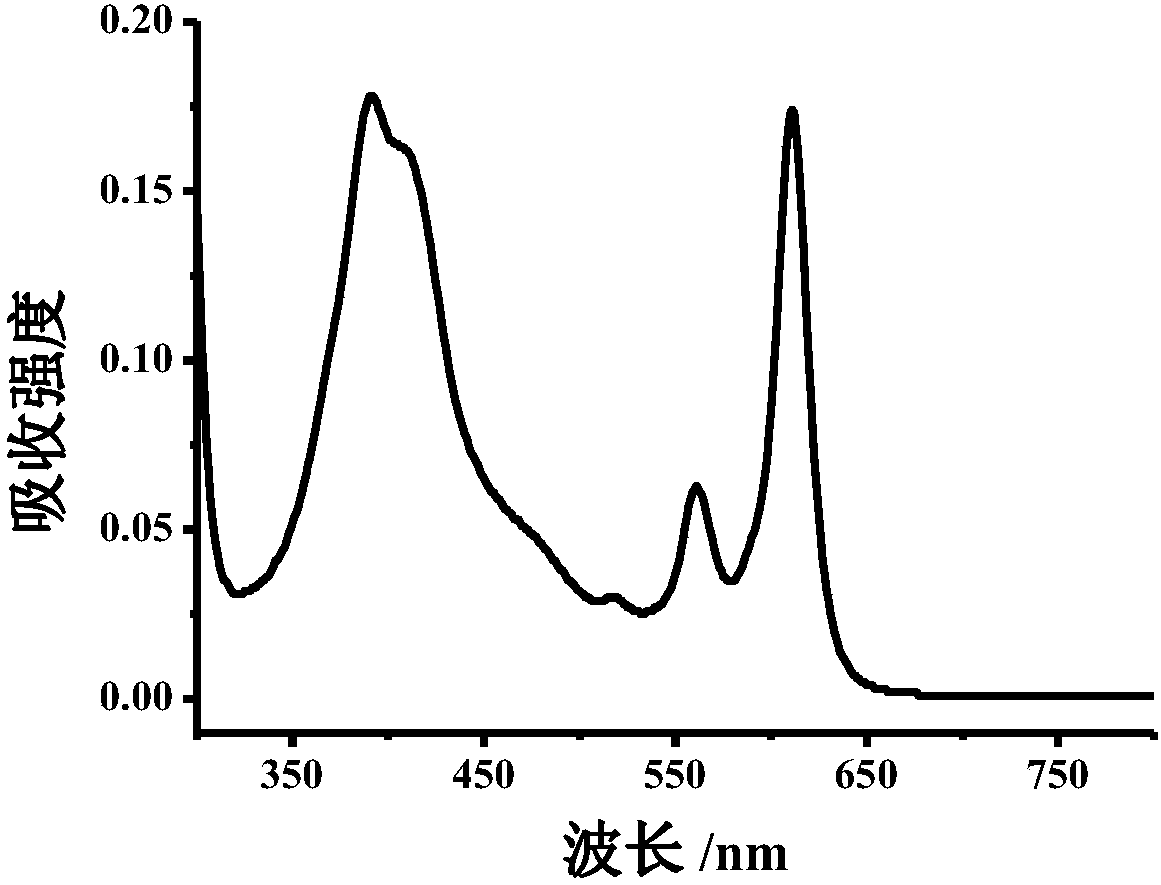
Method for detecting nitrite ions on basis of polymer carbon dot fluorescence colorimetry
ActiveCN108593618AEasy to synthesizeHighly sensitive and selective detectionFluorescence/phosphorescenceNitrite ionHigh selectivity
The invention belongs to the technical field of nanometer, and discloses preparation of polymer carbon dots and a detection method thereof applied to nitrite ion colorimetry fluorescence. When different concentrations of nitrite ions exist in a buffer system containing a certain amount of polymer carbo dots, for an absorption spectrum, the absorption peak in a 610nm position is obviously reduced;the absorption peak in a 380 nm position is gradually increased; the intensity of the fluorescence emission peak in a 625 nm position of the fluorescence spectrum is gradually reduced along with the increasing of the nitrite ion; Therefore according to the change of polymer carbon dot fluorescence spectrum and absorption spectrum in a system, through fluorescence colorimetry multi-output signal analysis, the high-sensitivity and high-selectivity detection on the nitrite ions can be realized; the detection on the nitrite ions in a complicated system such as urine can be further realized. A polymer carbon dot-based fluorescent colorimetric probe is a novel expansion on the original analysis method, and is hopeful to be used for the analysis and detection of nitrite ions in the complicated systems such as food, environment and the like.
Owner:NANJING UNIV OF TECH
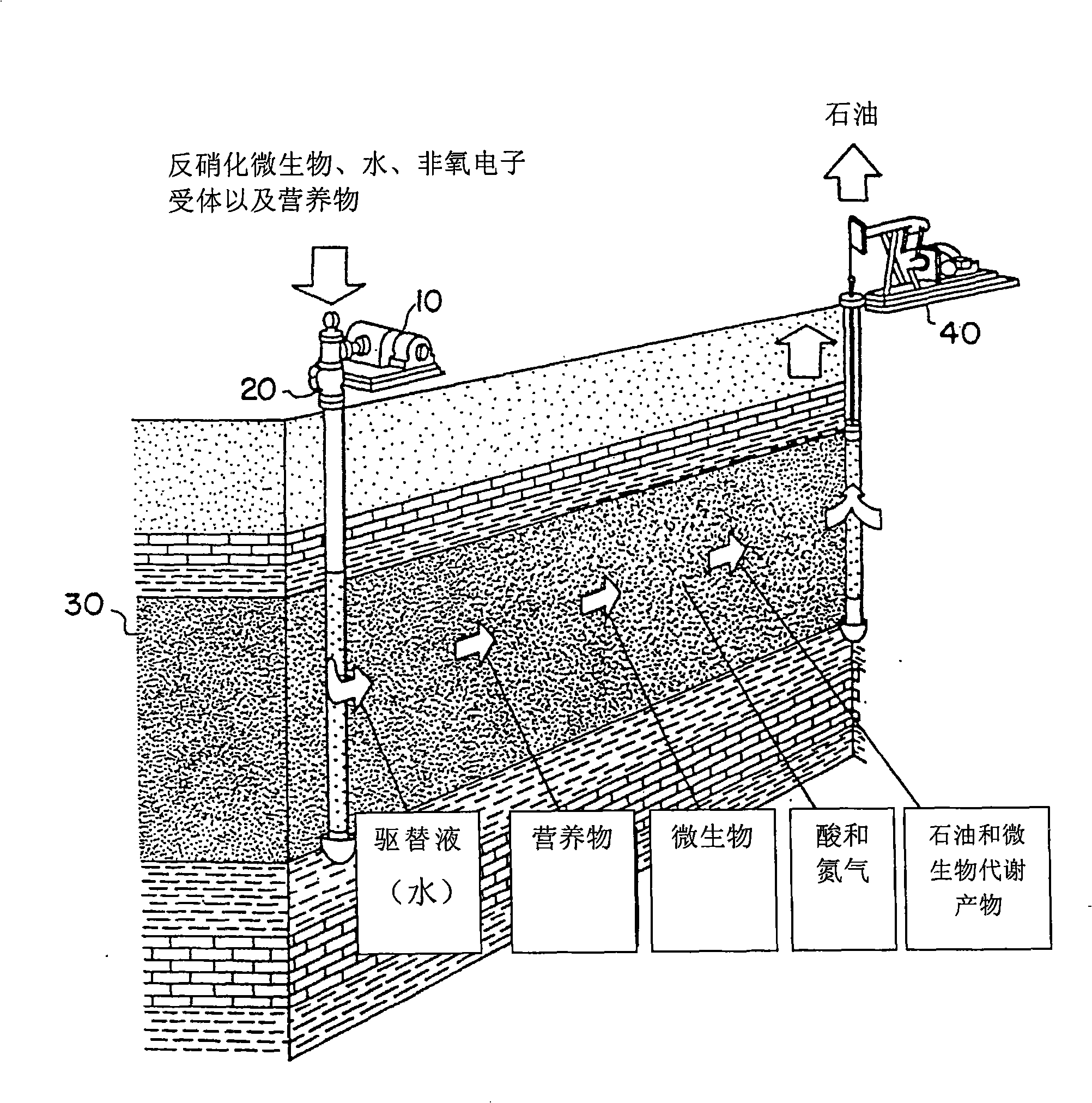
Method and preventing and reducing hydrogen sulfide of aqueous system and promoting oil recovery factor
The present invention provides a method for preventing and reducing sulfureted hydrogen in an aqueous system. The method not only can remove the sulfureted hydrogen in the aqueous system but also can effectively restrain sulfate reducing bacteria (SRB) from producing the sulfureted hydrogen by adding nitrate ion and / or nitrite ion. Nitrite and denitrifying microorganism can remove the sulfureted hydrogen in the aqueous system. Relative to the sulfate reducing bacteria (SRB), the denitrifying microorganism can more effectively use the prior carbon source and avoid the sulfate reducing bacteria(SRB) to produce the sulfureted hydrogen further. The present invention also provides a method for improving petroleum recovery ratio. An aqueous system that the sulfureted hydrogen is basically removed and the denitrifying microorganism exists is used for improving the petroleum recovery ratio by a microorganism oil increment system.
Owner:ADVANCED ENERGY & ENVIRONMENTAL TECH INC +1
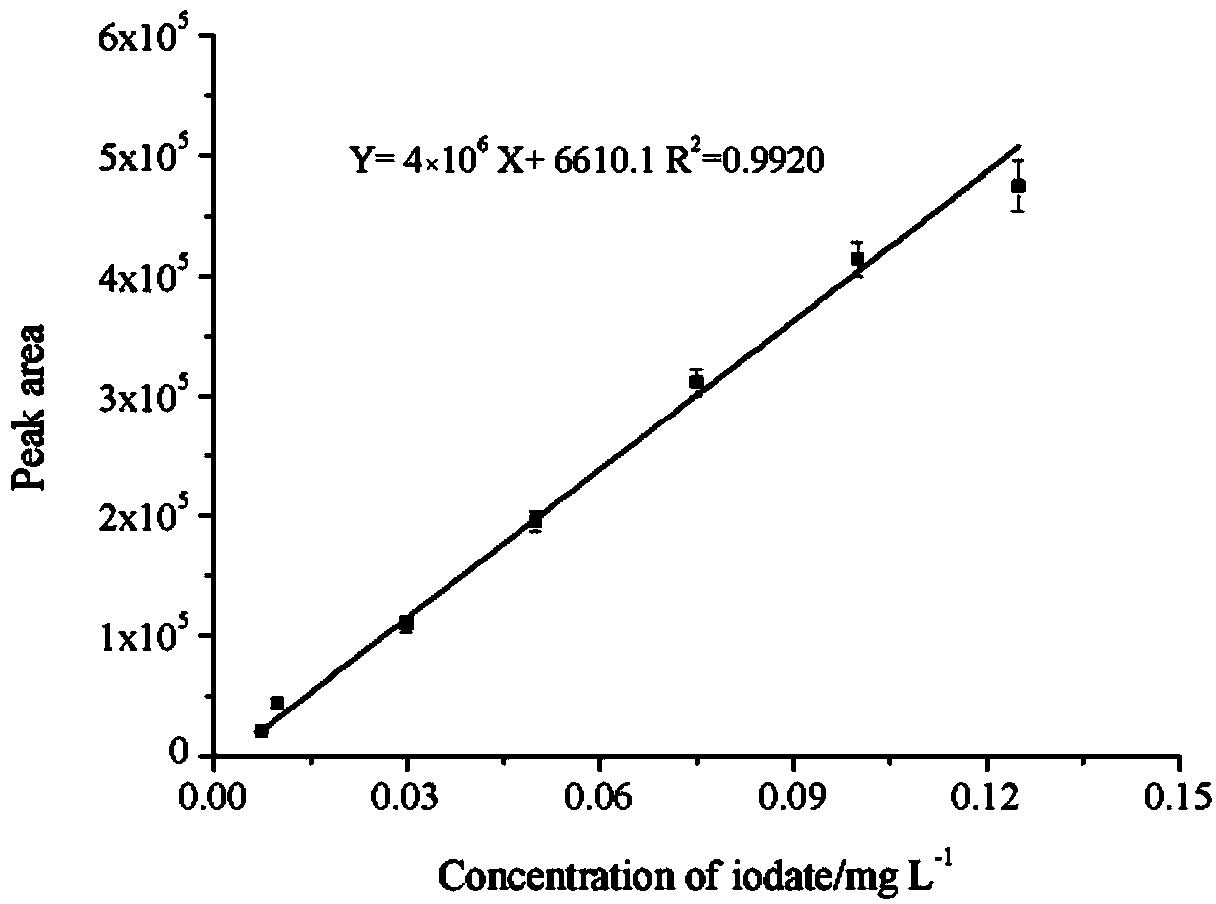
Method for rapidly detecting iodate by surface enhanced Raman spectroscopy and application of method
InactiveCN103411956AQuick checkApplicable to on-site inspectionRaman scatteringNitrite ionHydroxylamine
The invention relates to a method for rapidly detecting iodate by surface enhanced Raman spectroscopy and an application of the method. A Raman signal of the iodate is very weak, an SERS (Surface Enhanced Raman Scattering) signal of iodate is also weak, hydroxylamine is oxidated into nitrite by the iodate, coupling dye is generated from nitrite ions and a diazo-coupling reagent under an acidic or alkalescent condition, the SERS signal of the coupling dye is strong, and qualitative and quantitative detection of the iodate is indirectly carried out by measuring the dosage of the coupling dye through SERS. According to the method and the application thereof, Au / SiO2 is used as an SERS substrate material, the stability of particles is improved by means of a silicon dioxide shell, so that the result repeatability is better. The method is suitable for rapid detection of the iodate in complex samples, such as tablet salt, drinking water and environment water and has the advantages of simpleness and rapidness (about 2 minutes), suitability for field detection and benefits for popularization and use and the like.
Owner:SUN YAT SEN UNIV
Preparation method of graphite olefince modified electrochemical sensor electrode
InactiveCN102645468AQuick responseHigh sensitivityMaterial electrochemical variablesNitrite ionPhosphate
The invention relates to a preparation method of a graphite olefince modified electrochemical sensor electrode. The preparation method comprises the following steps of: preparing water solution of graphite olefince (GO); preparing pure glass carbon (GC) electrode; dropping the solution of the graphite olefince (GO) onto the surface of the glass carbon (GC) electrode, and drying the electrode at room temperature to obtain graphite olefince oxide modified glass carbon electrode which is marked as GO / GC electrode; and immersing the GO / GC electrode into 10mmol / L phosphate buffer with the pH value of 5 for potential scanning to prepare the graphite olefince modified electrode. As the graphite olefince electrode has high activity to the NO electrochemical reduction, the interference effect of nitrite ion (NO2-) and other biological substances can be neglected substantially in the presence of Nafion membrane. Therefore, the prepared electrode has rapid response to NO, has high sensitivity and good stability and can be applied to detecting the oxide of sweat nitrogen.
Owner:WUXI BAILING SENSING TECH
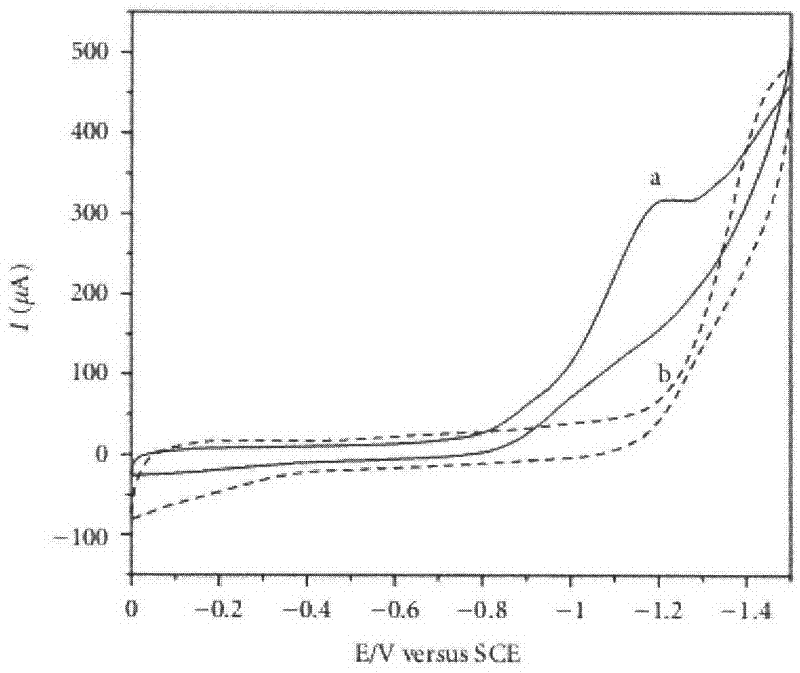
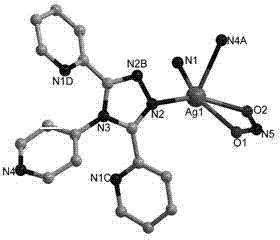
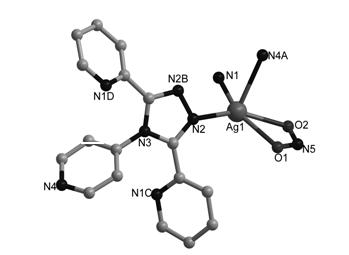
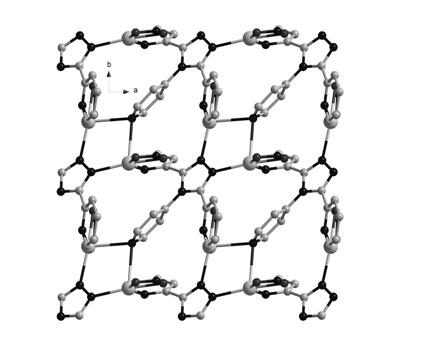
Metallic silver coordination polymer with two-dimensional lamellar structure, and preparation and application thereof
InactiveCN102516270AWith selective switchingEasy to manufactureAnion exchanger materialsSilver organic compoundsNitrite ionBenzoic acid
The invention relates to a 3,5-di(2-pyridyl)-4-(4-pyridyl)-1,2,4-triazolyl-silver (I) coordination polymer, and a preparation and application thereof. The compound is synthesized by the following steps: under normal temperature and pressure, mixing AgNO2 acetonitrile solution and ligand L chloroform solution, and keeping stirring for half an hour, and standing in a dark place to volatilize for about one week, thereby obtaining the colorless lumpy monocrystal product. The invention has the advantages of simple preparation technique, short reaction time, easy after-treatment and high yield. The experiment proves that nitrite ions in the material can selectively react with tetrafluoroborate ions, hexafluorosilicate ions, nitrate ions and perchlorate in an anion exchange mode, and benzoate ions and acetate ions can not perform similar anion exchange reaction. The material overcomes the limitation to the existing anion exchange material, the exchange process is simple and easy to implement, and thus, the invention is hopeful to practical application in the field of ion exchange materials.
Owner:TIANJIN NORMAL UNIVERSITY
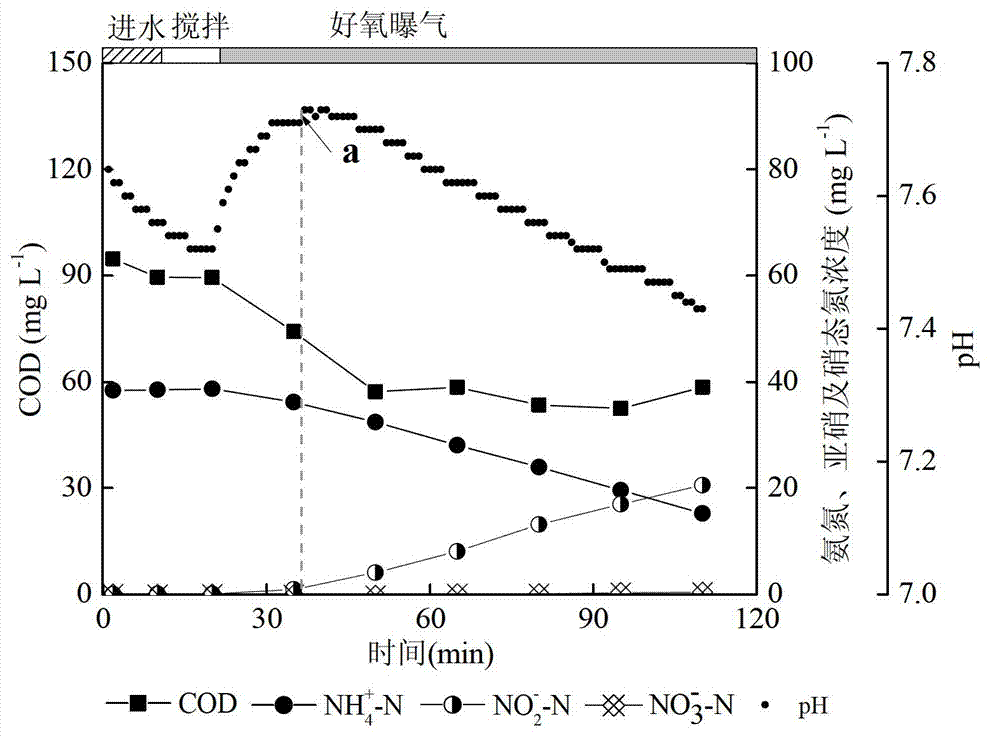
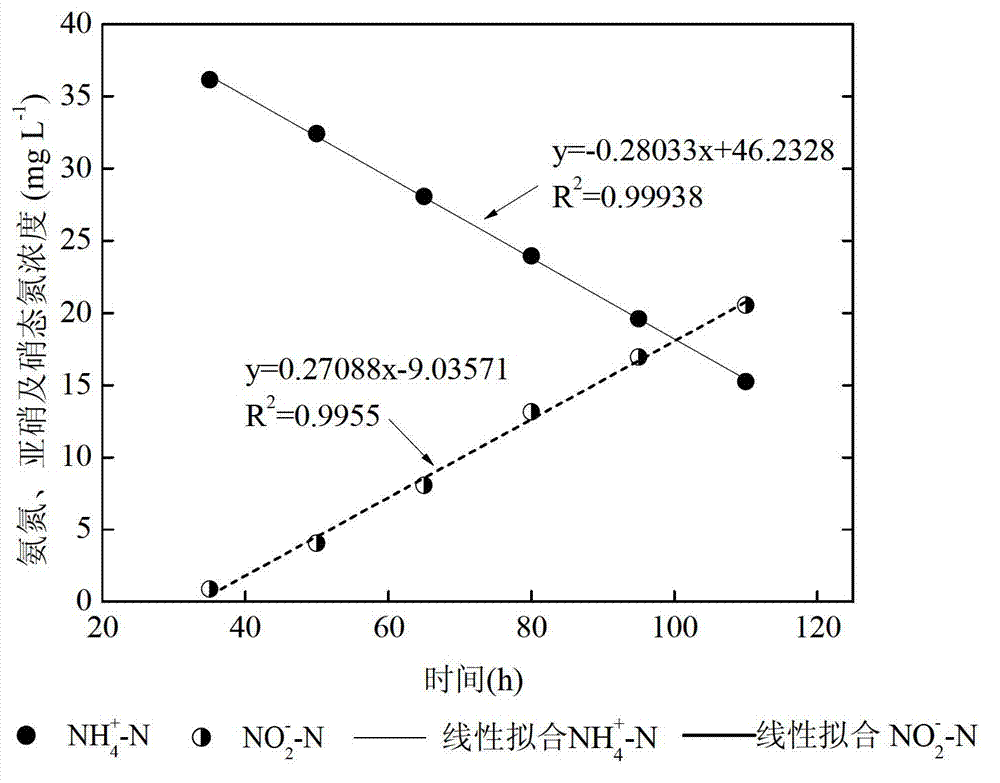
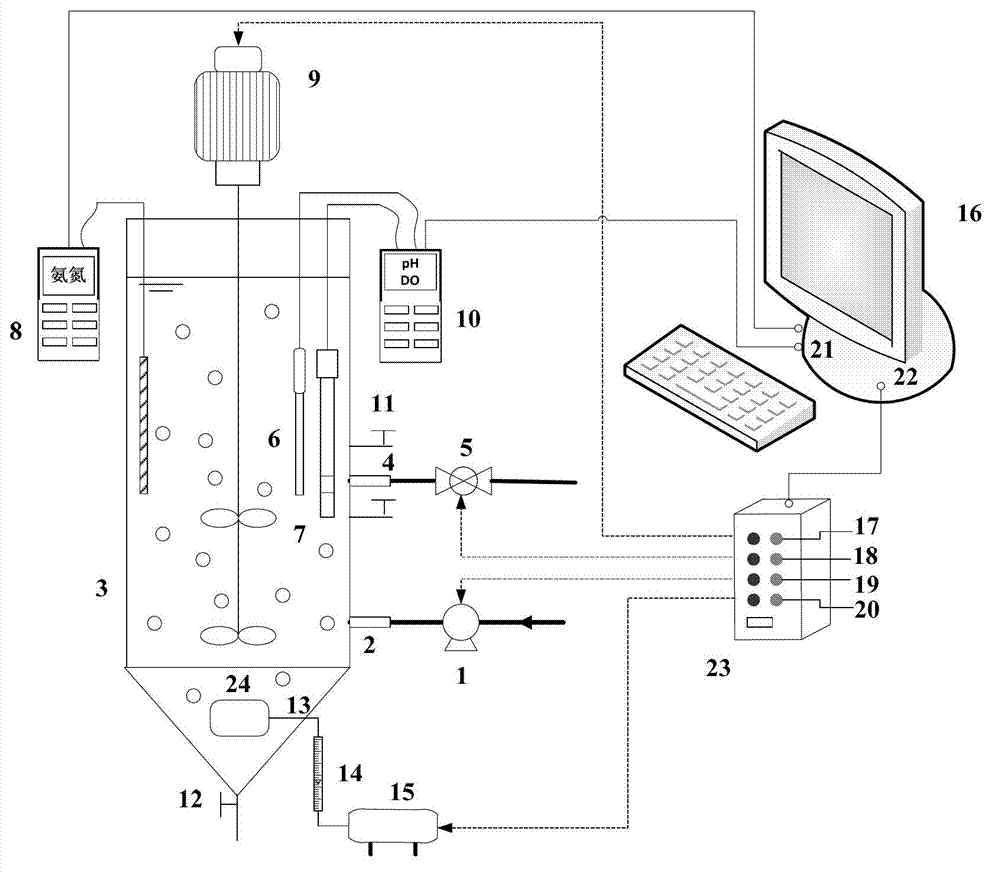
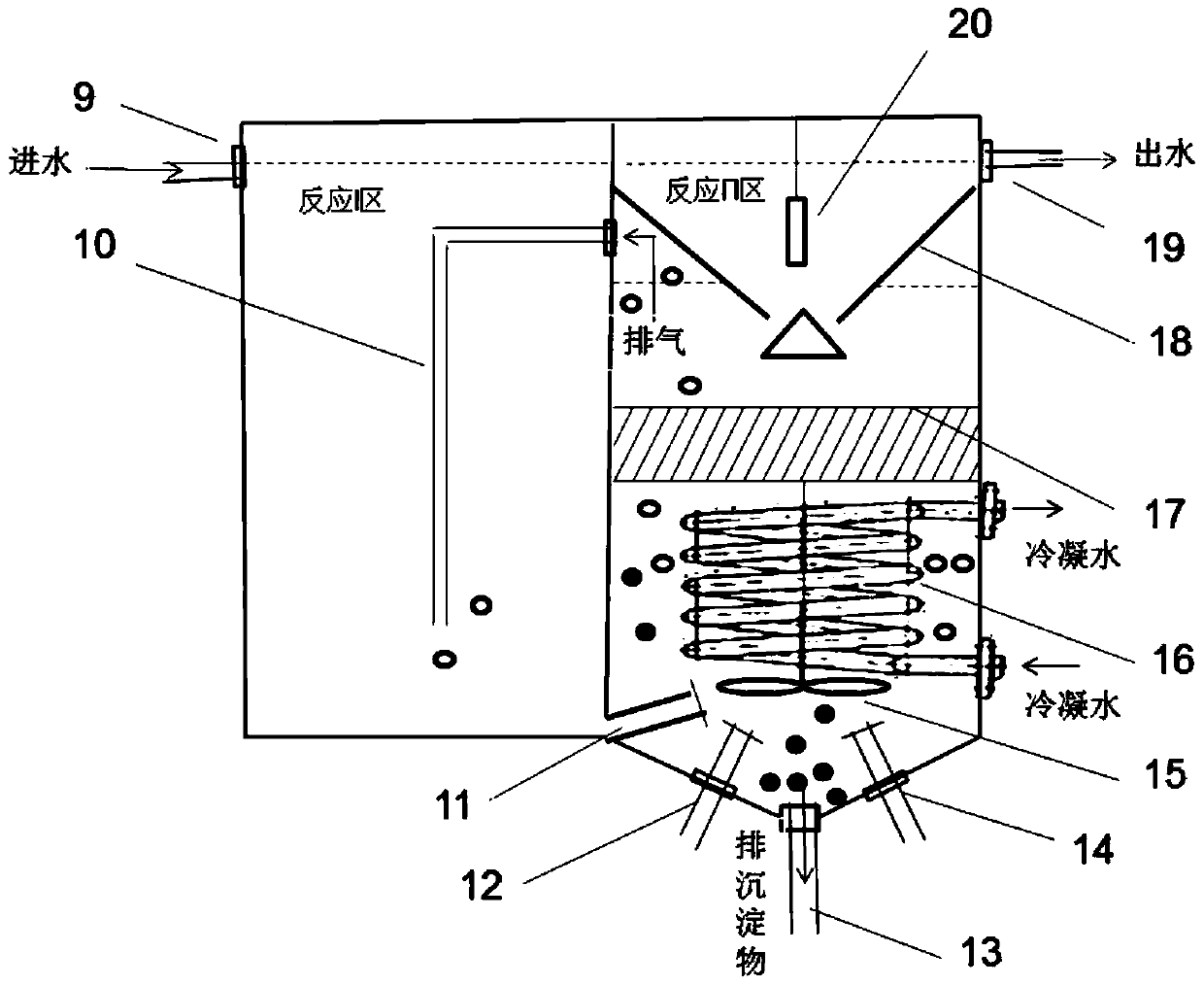
SBR (sequencing batch reactor) semi-short-distance nitrification process control method
ActiveCN103112949AGuaranteed uptimeEasy to operate and manageTreatment with aerobic and anaerobic processesNitrite ionSequencing batch reactor
The invention provides an SBR (sequencing batch reactor) semi-short-distance nitrification process control method, belonging to the technical field of biological sewage treatment and being applicable to semi-short-distance nitrification treatment of low-ammonia-nitrogen wastewater such as urban domestic sewage. The SBR semi-short-distance nitrification process control method comprises five stages, namely inflow stirring and inputting of initial ammonia concentration, aeration stirring, sedimentation, dewatering and standing, wherein an aerobic aeration time is calculated according to a formula ta=tCOD+0.56alphaS0 / ((S0-Sn) / (n-tCOD)), aeration is timely stopped, and semi-short-distance nitrification is realized, wherein concentration ratio of nitrite ions to ammonia nitrogen in yielding water is maintained to be 0.9-1.5; and process management and operation are easy, and impact resisting load of s system is high. Compared with the traditional SBR process control technique, a semi-short-distance nitrification reactor can maintain high nitrite accumulation rate more stably; and the problem that the concentration ratio of the nitrite ions to the ammonia nitrogen in the yielding water of semi-short-distance nitrification can not be controlled in a domestic sewage biological treatment technology is solved, and a necessary inflow guarantee is provided for stable operation of an autotrophic denitrification system in the urban sewage low-ammonia-nitrogen wastewater.
Owner:BEIJING UNIV OF TECH
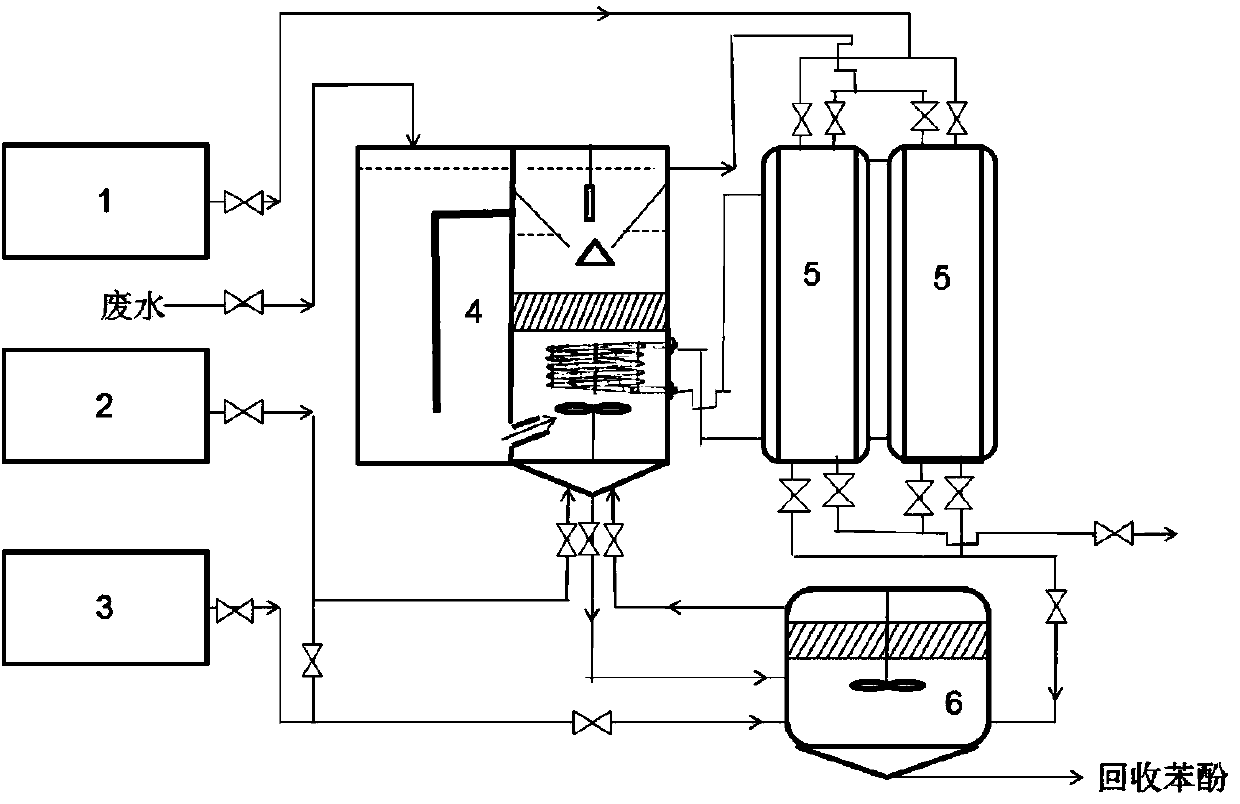
Condensation mother liquid waste water pretreatment and resource system and method in compound neutralization reactor in disperse blue 56 production process
ActiveCN103588329AReduce dosageAvoid harmOrganic chemistryOrganic compound preparationLiquid wasteNitrite ion
The invention discloses condensation mother liquid waste water pretreatment and a resource system and a method in a compound neutralization reactor in a disperse blue 56 production process, which belongs to the waste water treatment field. The method comprises the following steps: highly basic condensation mother liquid waste water in the disperse blue 56 production process and a mixed acid solution (sulfuric acid and sulfamic acid) in the compound neutralization reactor are neutralized, three purposes of reducing the pH value of waste water, precipitating phenol and removing nitrite can be simultaneously realized; the neutralized waste water passes through a fixed bed adsorption column filled with adsorption resin, so that residual phenol in the waste water can absorb on the resin column. Resin after adsorption saturation is carried out with desorption and regeneration by using a NaOH solution and then the resin is repeatedly usable, and the desorption liquid passes through the processes of acidity adjusting and vacuum distillation of phenol to realize recycling of phenol. According to the condensation mother liquid waste water which is pretreated in the disperse blue 56 production process, CODCr value is decreased from about 55000mg / L to less than 500mg / L, the phenols substance concentration is decreased from about 19000mg / L to less than 20mg / L, the nitrite ion is decreased from 87000mg / L to less than 20mg / L; thereby the treatment and resource utilization of waste water can be effectively realized.
Owner:江苏吉易达环保科技有限公司
Thermal energy storage materials
The present invention relates to a thermal energy storage material (TESM) system (and associated methods) that reproducibly stores and recovers latent heat. The Thermal energy storage material system comprises i) at least one first metal containing material including at least one first metal compound that includes a nitrate ion, a nitrite ion, or both; and ii) at least one second metal containing material including at least one second metal compound. The thermal energy storage material system may water. If any water is present in the thermal energy storage material system, the water concentration should be less than about 10 wt. %. The thermal energy storage material has a liquidus temperature, TL, from about 100° C. to about 250° C. and exhibits a heat storage density from 300° C. to 80° C. of at least about 1 MJ / l, so that upon being used in a system that generates heat, at least a portion of the heat is captured and stored by the thermal energy storage material and subsequently released for use. The thermal energy storage material system is generally resistant to corrosion at temperatures of about 300° C. Exemplary metal compounds include one or more cations selected from the group consisting of Li, Na, K, Be, Mg, Ca, Al, and Ga.
Owner:DOW GLOBAL TECH LLC
Method for preparing catalyst for refining of crude terephthalic acid
ActiveCN100402144CLower redox potentialRestore will notOrganic compound preparationCarboxylic compound preparationNitrite ionPhenanthroline
This is a kind of producing method of catalyst used for refining coarse terephthalic acid (CTA). The catalyst makes grains or molding active carbon as carrier, the content of loading active component metal Pd is 0.2 - 4wt%. It orderly includes: have acid wash to carrier active carbon; wash carrier active carbon with water to neutral; have soakage with watery solution containing nitrite ion to carrier active carbon; dry carrier active carbon to get rid of water; prepare Pd solution with water-soluble Pd compound, a kind of complexing agent and water to infuse or insufflate the carrier active carbon; the content of Pd compound in the Pd solution counted by Pd is 15 - 20wt%; the mol proportion of complexing agent and Pd is (0.01 - 1) : 1, the complexing agent is got from anyone of 8-hydroxyquinoline, 2, 3, 4-trihydroxyl-4-sulfonic azobenzol, o-phenanthroline, o-Aminophenol, o-hydroxybenzoic sodium or o-hydroxybenzaldehyde oxime; after aginged, have deoxidizing treatment with reducing agent to get activator products.
Owner:CHINA PETROLEUM & CHEM CORP
Method for determining nitrate and nitrite ions in cigarette tipping paper
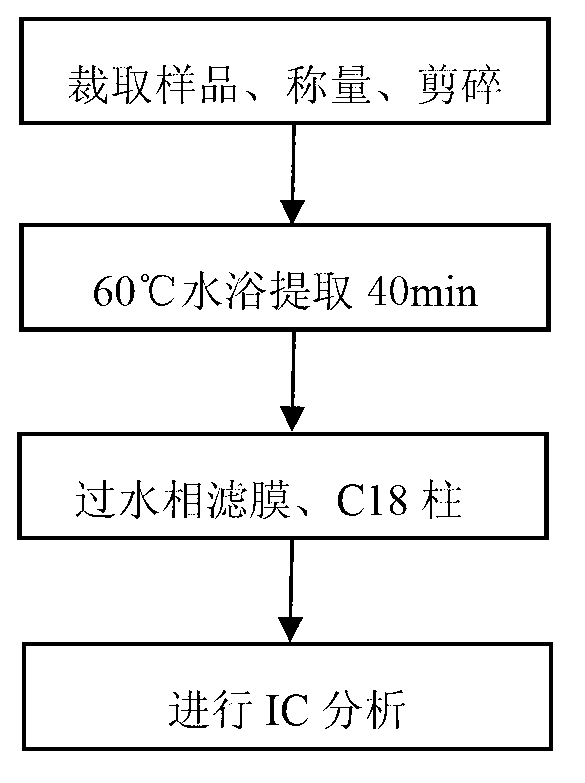
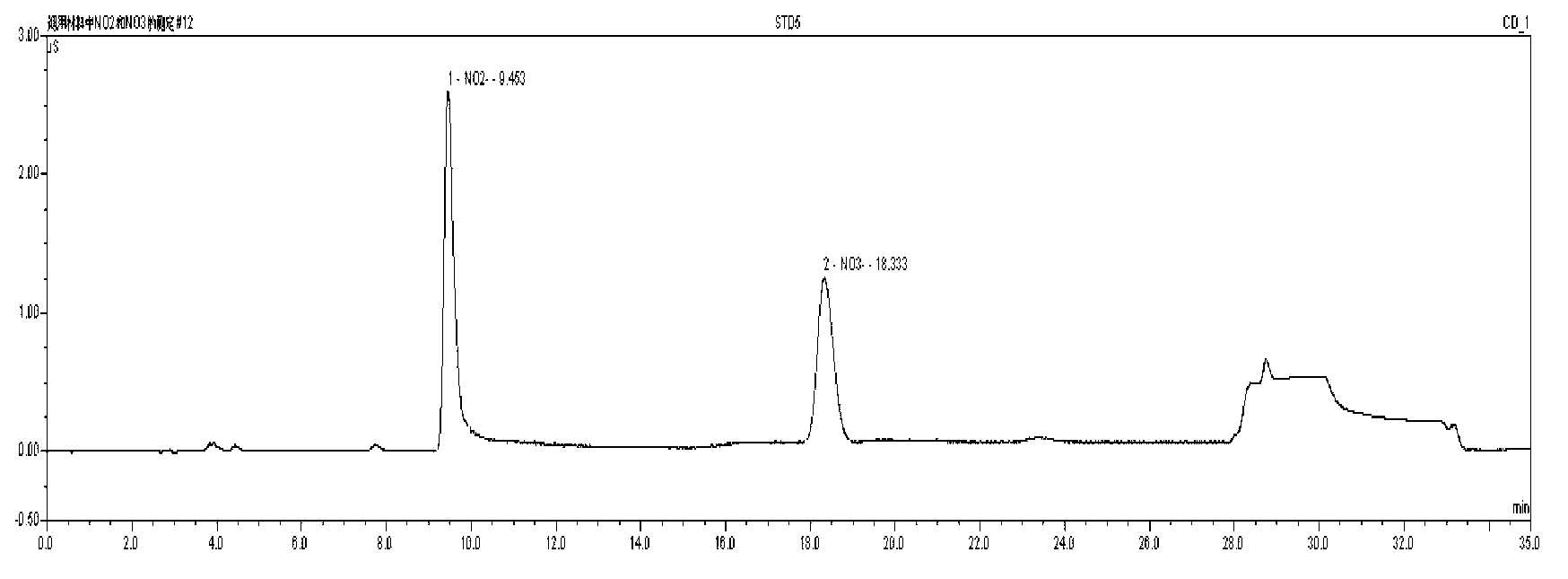
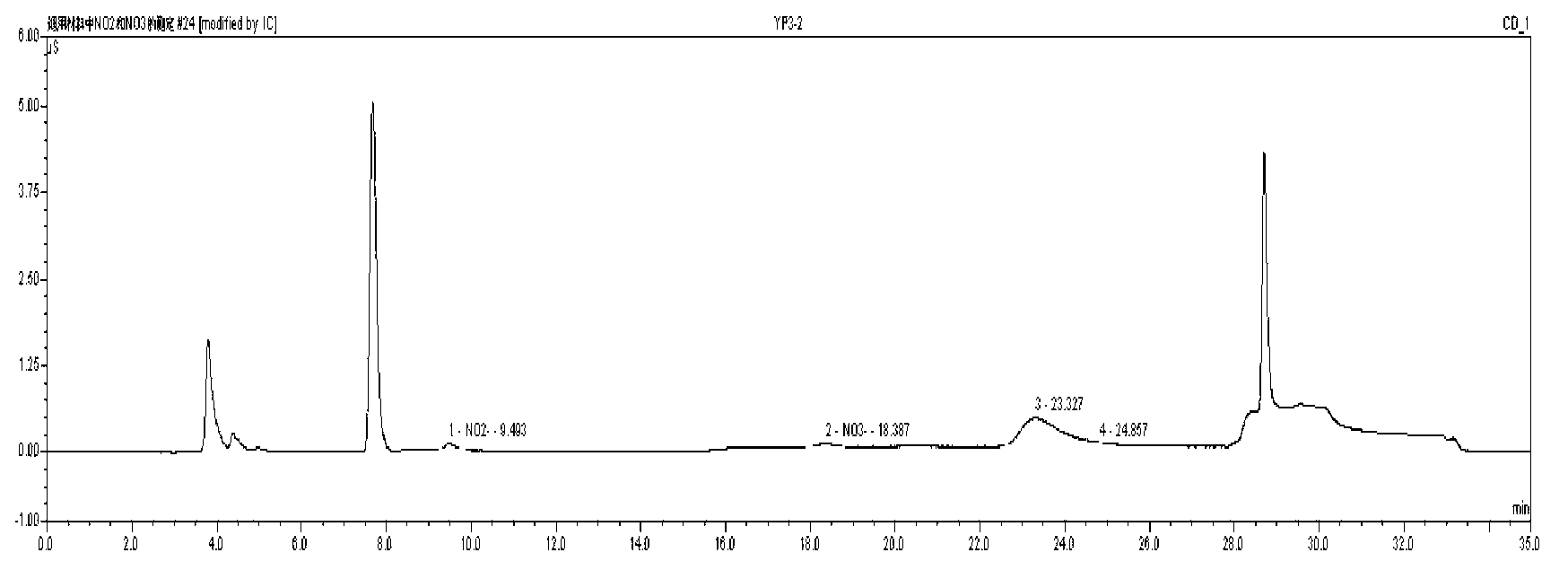
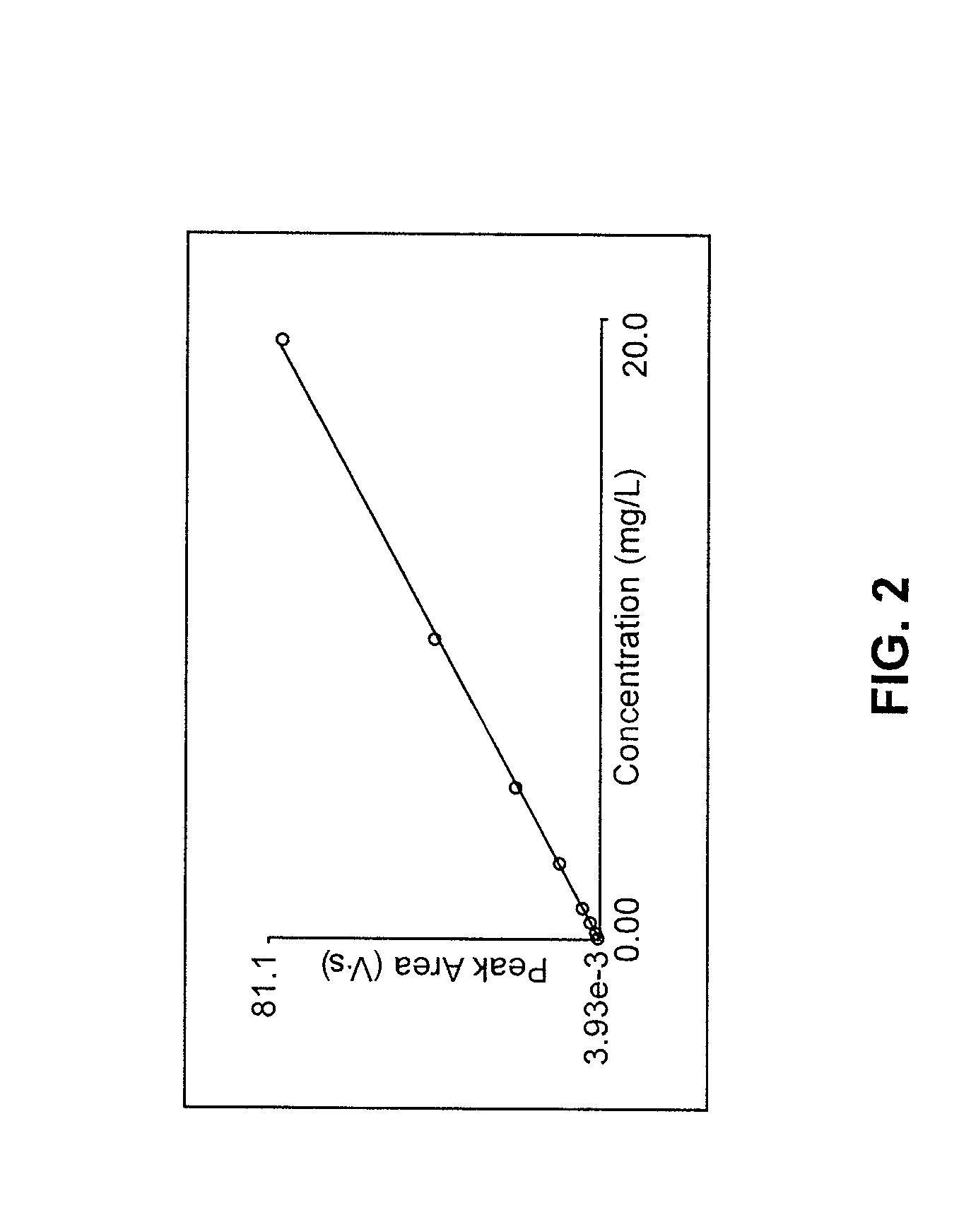
The invention discloses a method for determining nitrate and nitrite ions in cigarette tipping paper. The method consists of: cutting a tipping paper sample and weighing it, dipping sample fragments in deionized water, conducting water bath extraction, then carrying out static cooling to room temperature; taking the supernatant, and passing it through a water-based filter membrane syringe needle filter and a C18 column, thus obtaining a sample solution; weighing a nitrate ion standard solution and a nitrite ion standard solution, adding deionized water to a constant volume, and then implementing dilution with deionized water so as to obtain standard working solutions; then implementing ion chromatograph (IC) analysis on the standard working solutions and the sample solution to measure the peak areas of nitrate and nitrite ions in the sample, and substituting the peak areas into standard curves so as to calculate the content of nitrate and nitrite ions in the sample. The quality can be determined by standard substance retention time, and the quantity can be determined by the peak areas. The method can accurately determine the content of nitrate and nitrite ions in a cigarette tipping paper sample, has the advantages of rapidity, reliability, simplicity and practicability, and fills in the blank of determination of nitrate and nitrite ions in cigarette tipping paper at present.
Owner:HONGYUN HONGHE TOBACCO (GRP) CO LTD
Peroxynitrite ion detection probe as well as preparation method and application thereof
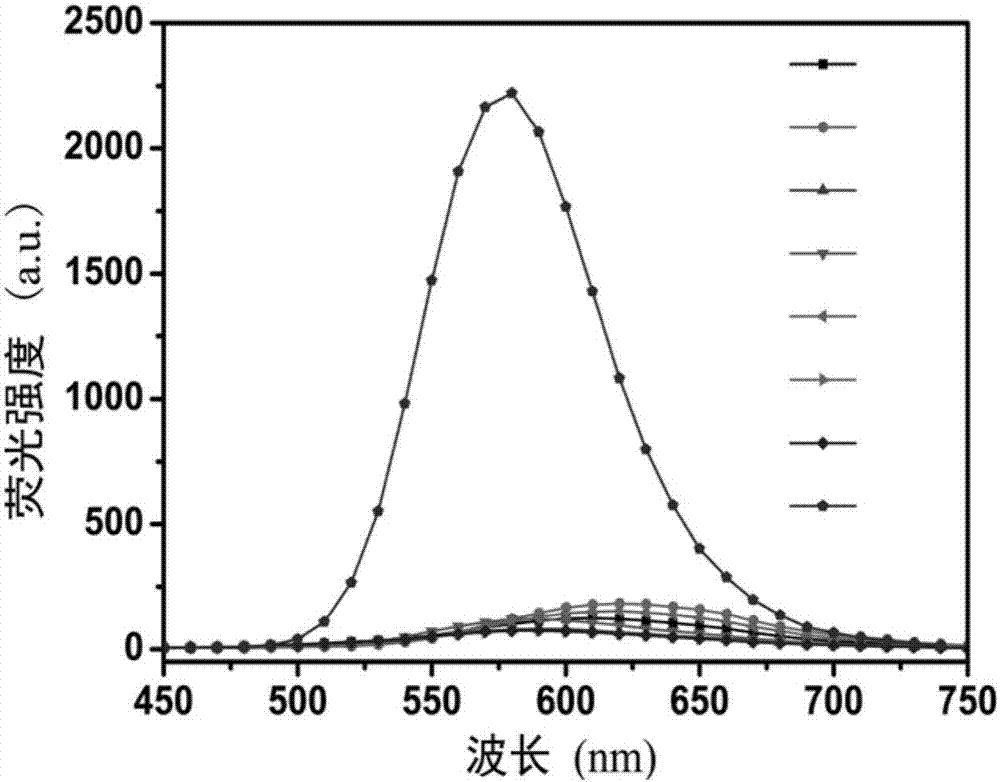
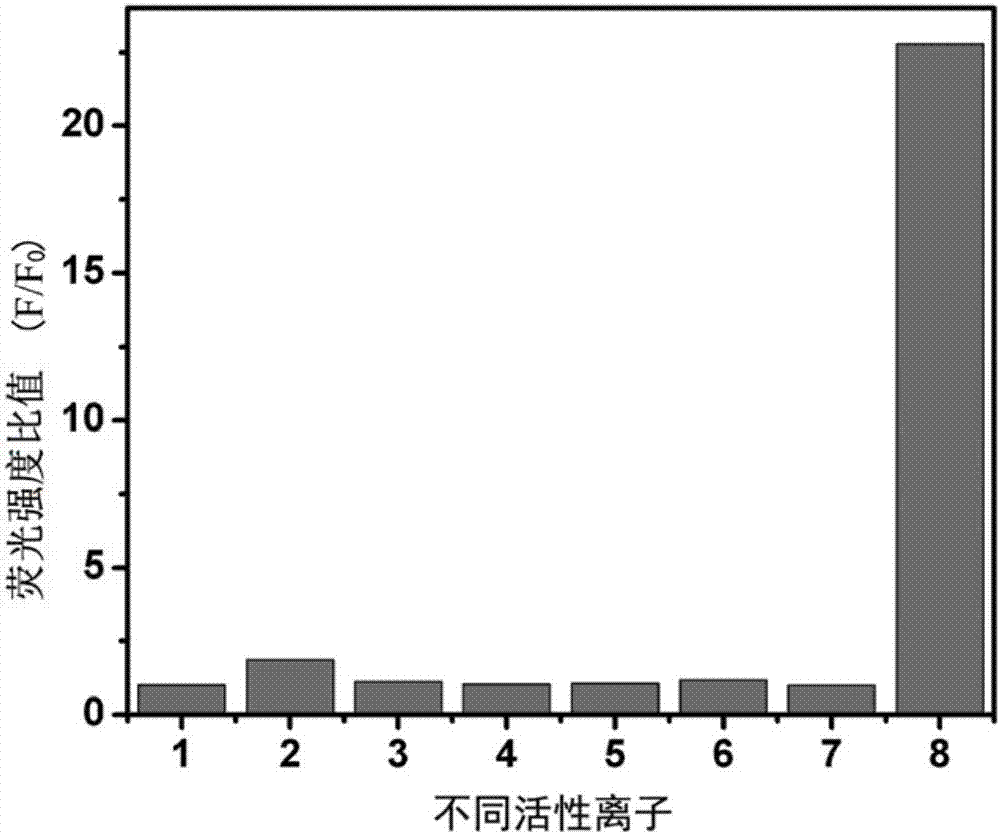
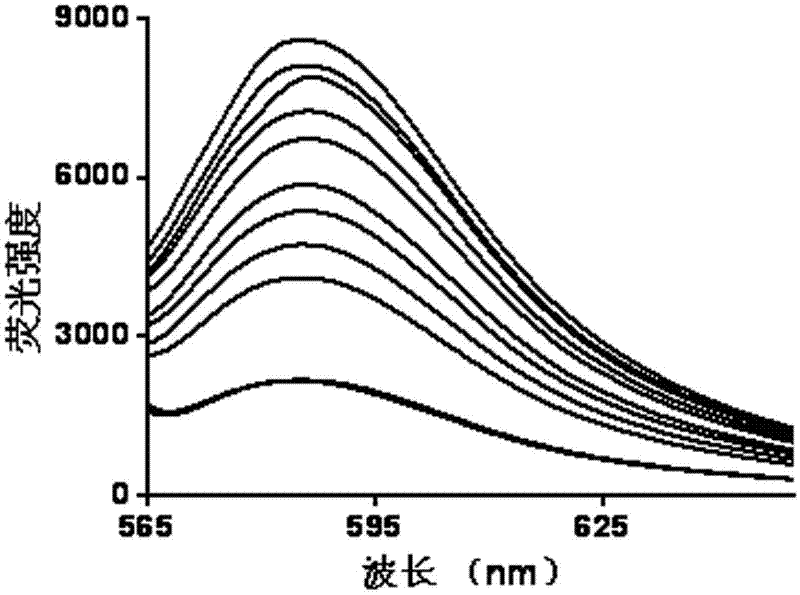
ActiveCN105866083ALuminous intensityImprove stabilityFluorescence/phosphorescenceNitrite ionFluorescence
The invention discloses a peroxynitrite ion detection probe as well as a preparation method and application thereof. The peroxynitrite ion detection probe comprises a fluorescent nano material and an enzyme, wherein the enzyme is combined with the fluorescent nano material, has an oxidation-reduction property and can be selectively oxidized by peroxynitrite ions to form an oxidized enzyme; after the fluorescent nano material is radiated by excited light, electrons in an excited state can be transferred to the oxidized enzyme and cannot turn back to a base state, and then fluorescent light of the fluorescent nano material can be quenched. As an inorganic light-emitting material is adopted as fluorophore, the peroxynitrite ion detection probe disclosed by the invention is intense in light emission, good in stability and less prone to photo-bleach; the surface of the probe is modified with the enzyme with oxidation-reduction property, and the enzyme can be selectively oxidized by ONOO<->, so that the probe is not interfered by other active oxygen (ROS) or active nitrogen (RNS), and is excellent in specificity.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Method for removing nitrite ions
InactiveCN101948388ADoes not affect product qualityNo follow-upOrganic chemistryOrganic compound preparationNitrite ionNitrate
The invention belongs to the technical field of environment protection, particularly relating to a method for removing nitrite ions. The method comprises the following steps: adding nitrified crude containing the nitrite ions into water to be fully stirred, or adding waste water containing the nitrite ions into water to be fully stirred; measuring the content of nitrous acid and nitric acid in an aqueous phase layer or waste water; and adding sulfamic acid into the aqueous phase layer or the waste water to cause the molar ratio of total amount of nitrous acid and nitric acid to sulfamic acid to be 1:1-1.5, wherein stirring time is at least 0.5 hour. The method breaks the nitrite ions generated by nitrification, neutralization and washing with sulfonamides so as to solve the technical problems that the original neutralization-urea washing-rinsing technology has high nitrite ion content in nitrate and can not reach the requirements of products, and waste water amount generated by multiple washing is large, is easy to pollute environment and the like.
Owner:ZHEJIANG KANGFENG CHEM
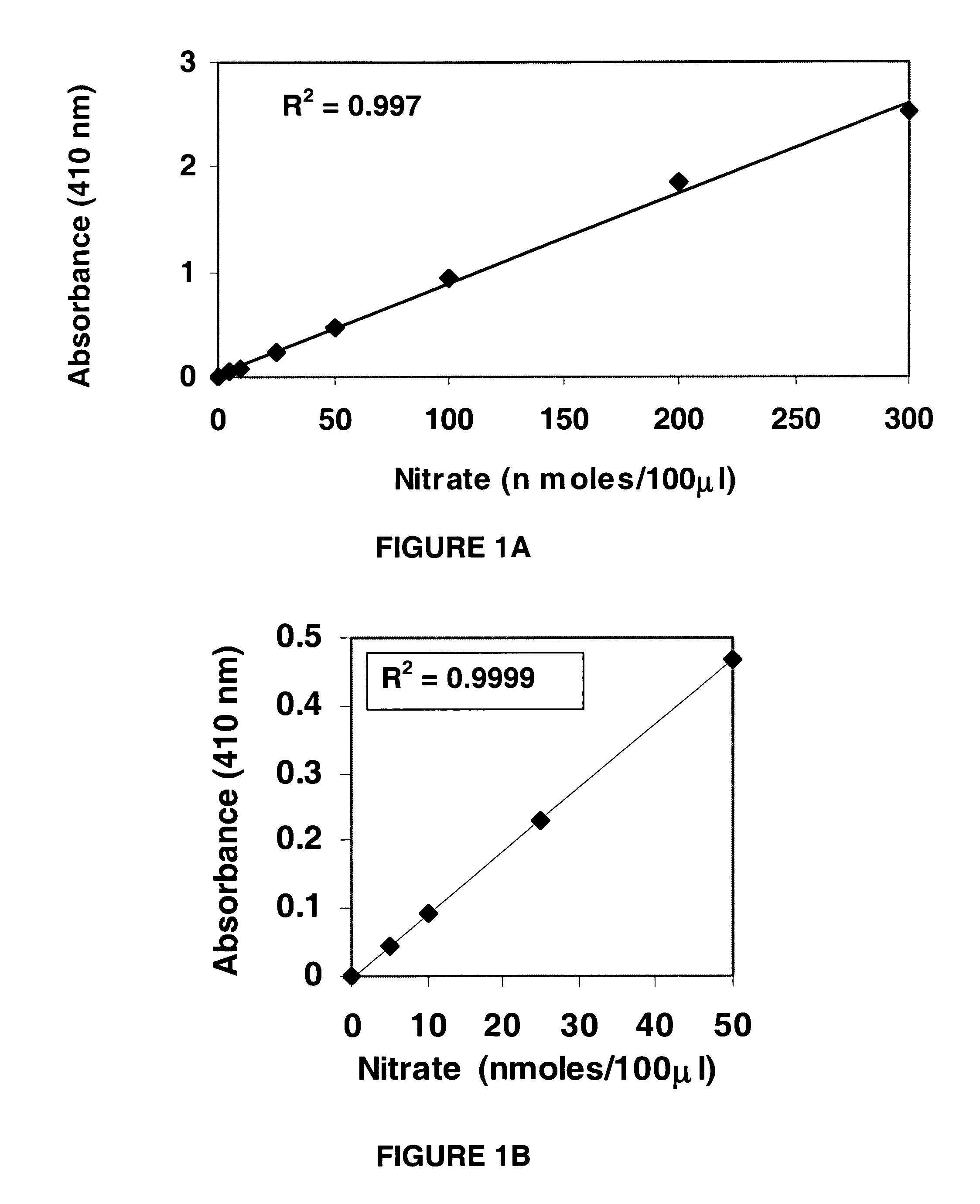
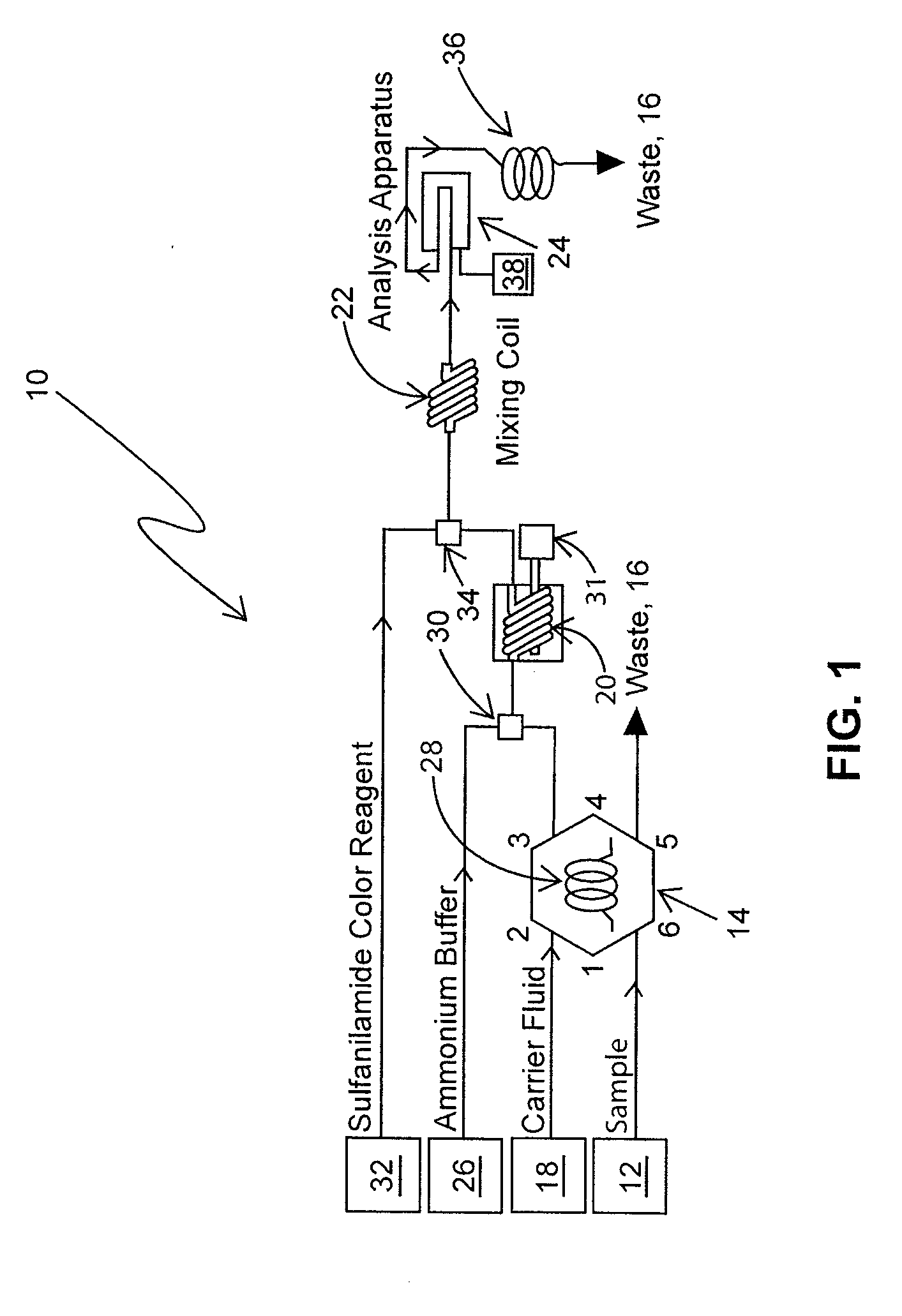
Nitrate/nitrite assay reagents, kit, and method of use
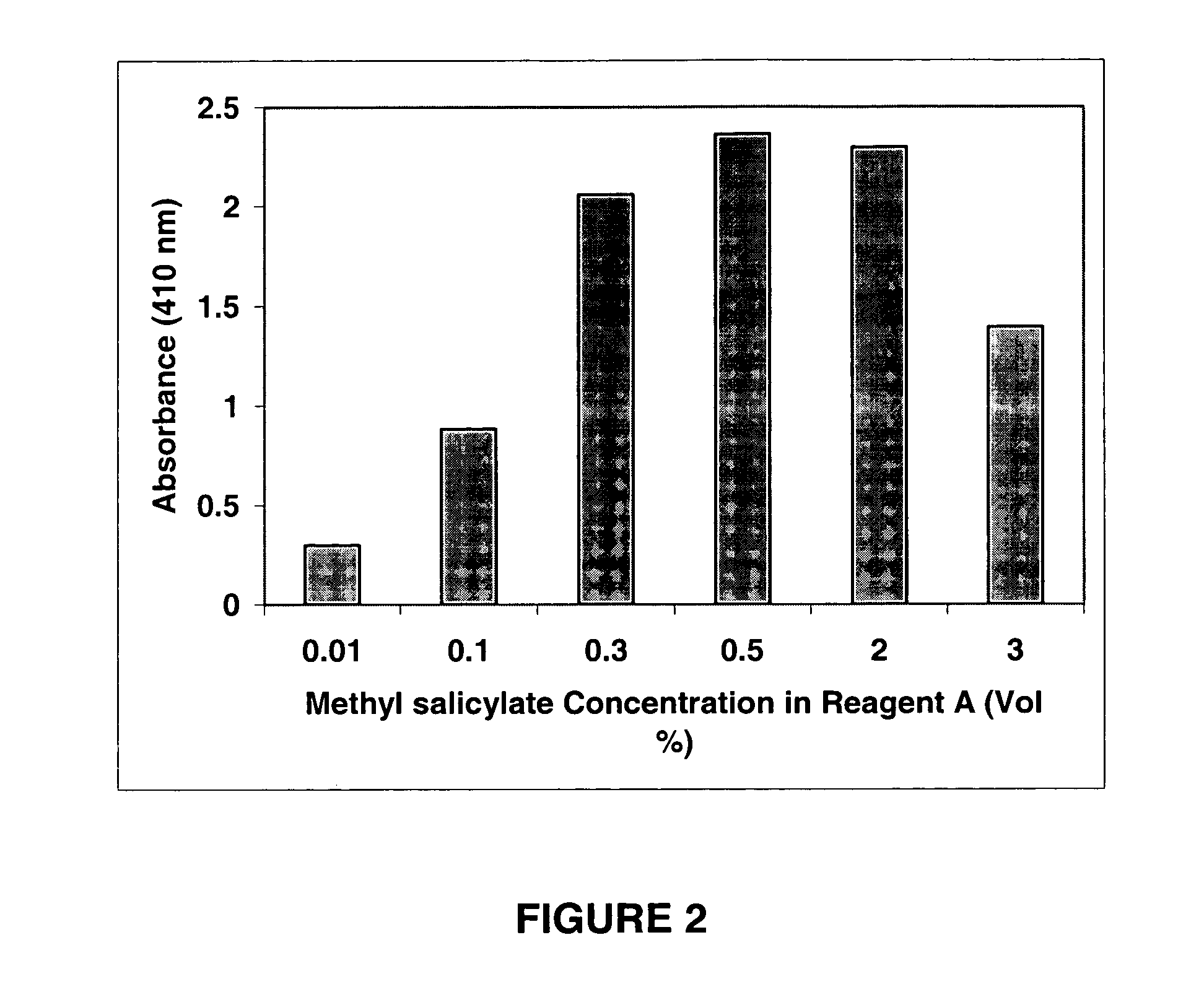
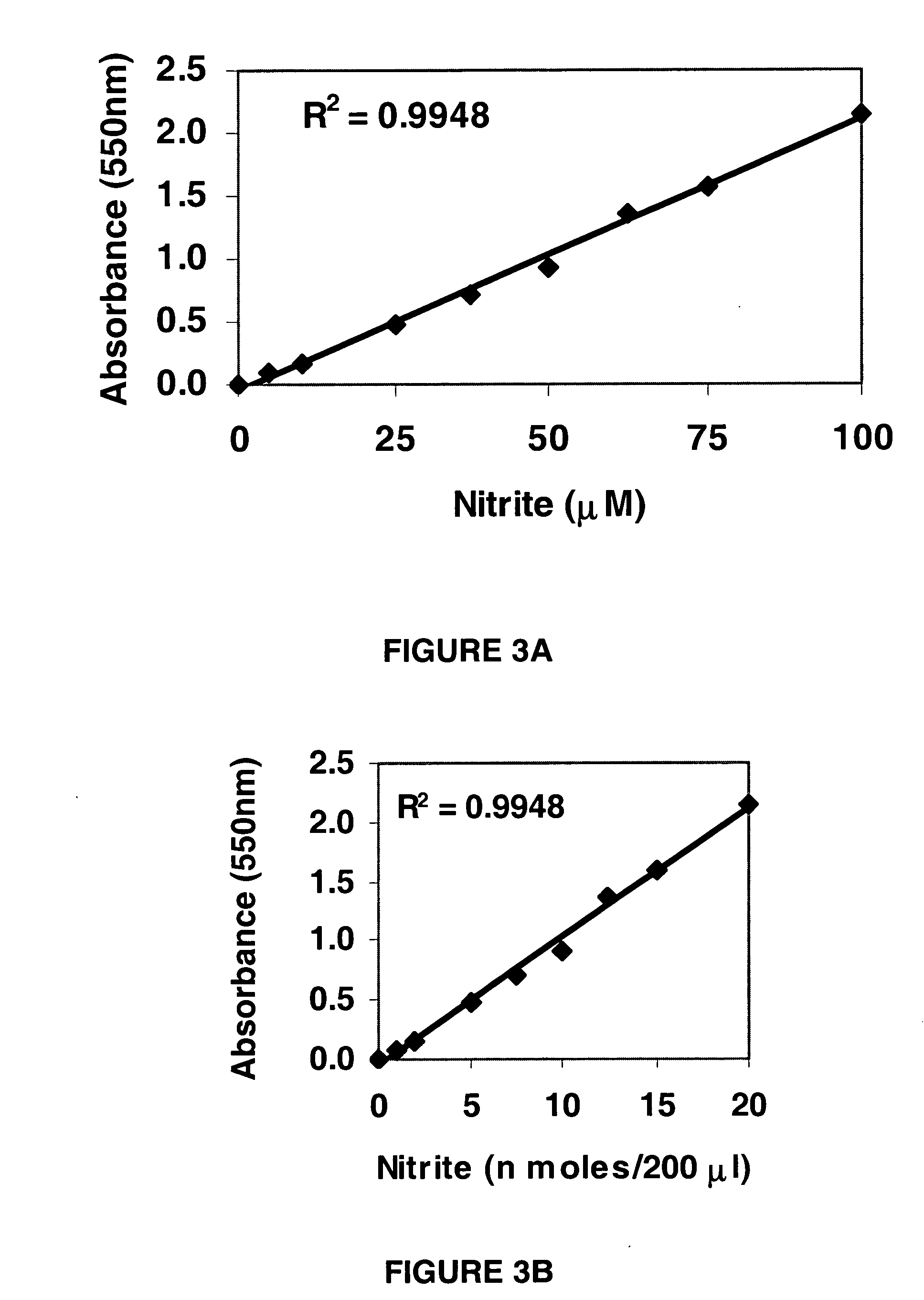
InactiveUS20060121621A1Rapid, reproducible and sensitiveBiological testingChemical methods analysisNitrite ionMethyl salicylate
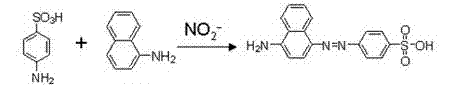
An assay kit and method for the quantitative and direct determination of the concentration of nitrate and nitrite ions in a sample solution. The nitrate assay is based on the reaction of methyl salicylate with nitrate ions to form 5-nitro-2-hydroxymethylbenzoic acid, which has a yellow color having a maximum absorbance of λ=410 nm. The nitrite assay is a derivative of the well-known Greiss diazotization reaction.
Owner:TS POLYMERS
Application of rhodamine B derivative in nitrite ion detection
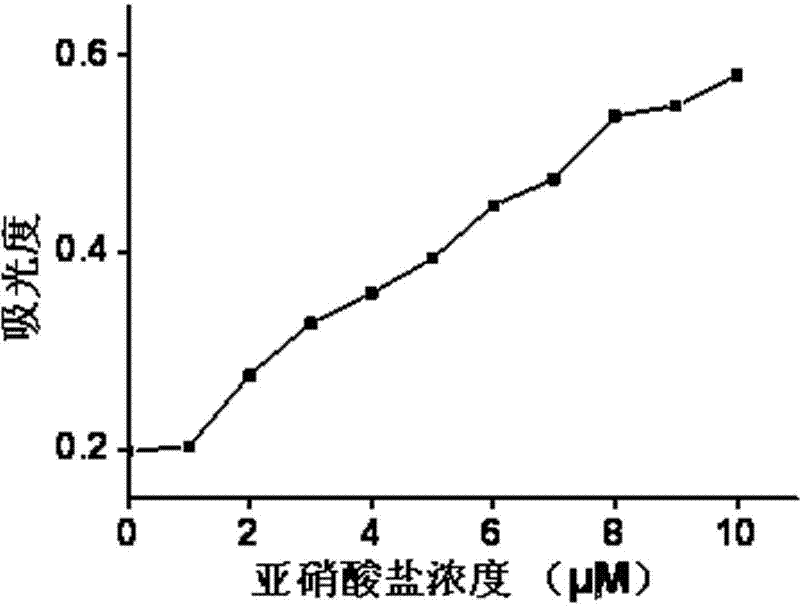
InactiveCN102519926ARapid responseLow detection limitColor/spectral properties measurementsFluorescence/phosphorescenceNitrite ionFluorescence
The invention discloses an application of a rhodamine B derivative in nitrite ion detection, and the rhodamine B derivative is the rhodamine B derivative by connecting five-membered spirolactam in molecule with 1,2-phenylenediamine. The rhodamine B derivative for nitrite detection is characterized in that 1,2-phenylenediamine is reacted with nitrite under acidic condition, and ring opening of the the rhodamine five-membered spirolactam in molecule is initiated, and a substance with the magenta color and strong fluorescence is generated. The invention has the beneficial effect that intermolecular five-membered rhodamine B spirolactam-o-phenylendiamine is reacted with nitrite to generate the substance possessing color and emission fluorescence. The color development reaction can be used in the fields that 1) nitrite existence can be visually measured; 2) the measurement of the fluorescent emission intensity or visible light absorption intensity of the reaction system through an analysis apparatus enables quantitative determination of the content of the nitrite in the sample.
Owner:XIAMEN UNIV
Preparation method of electrochemical sensor based on polyaniline nanofiber
InactiveCN102645461AImprove electrocatalytic activityHigh sensitivityMaterial electrochemical variablesNitrite ionNanofiber
The invention relates to a preparation method of an electrochemical sensor based on polyaniline nanofiber. The preparation method comprises the following steps of: firstly, preparing polyaniline nanofiber; then, dispersing the purified polyaniline nanofiber under ultrasonic oscillation into deionized water to form green suspension; and coating 10uL of water solution of the polyaniline nanofiber on the surface of a glass carbon electrode, and drying to obtain a modified electrode so as to prepare the electrochemical sensor based on polyaniline nanofiber. The sensor prepared by the invention shows high electro-catalysis activity to the reduction of nitrite ion, has high sensitivity, wide linear range, low detection limit and good stability, and provides a new way for monitoring the reaction process of ascorbic acid and nitrite ion.
Owner:WUXI BAILING SENSING TECH
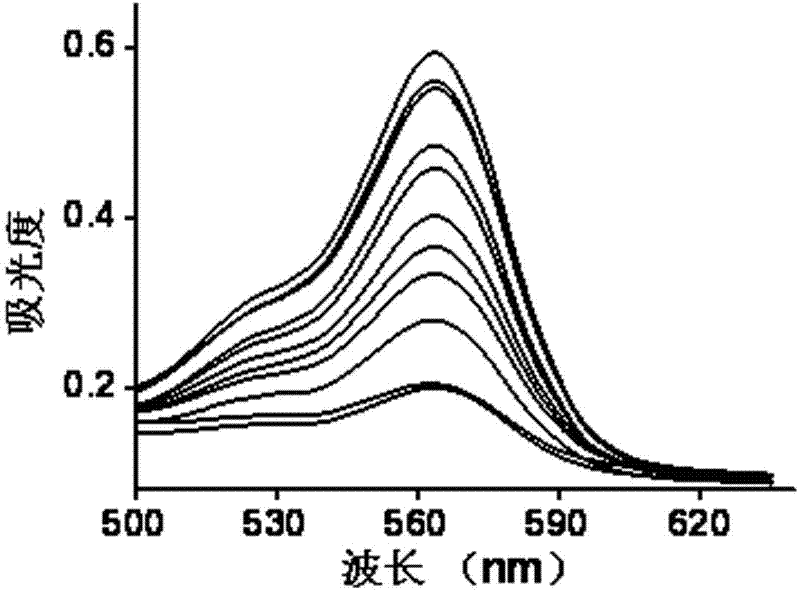
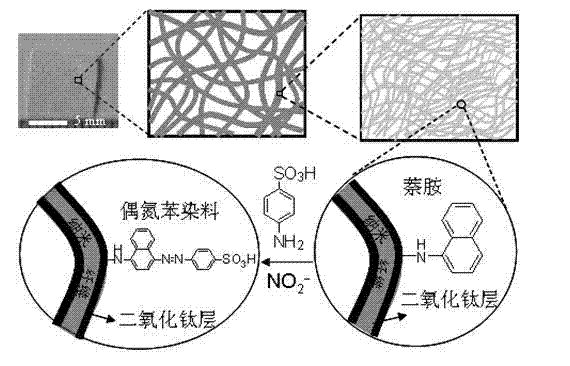
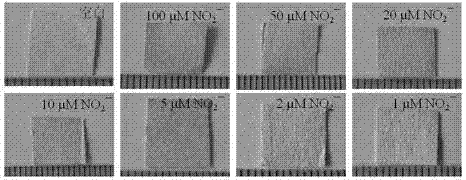
Preparation method of nitrite ion colorimetric sensing cellulose material
InactiveCN102175680AObvious color changeHigh sensitivityMaterial analysis by observing effect on chemical indicatorCelluloseNitrite ion
The invention relates to a preparation method of a nitrite ion colorimetric sensing cellulose material, comprising the following steps of: depositing a titanium dioxide film on the surface of filter paper fiber by taking a natural cellulose material as a substrate and tetrabutyl titanate as a precursor and using a surface sol-gel method; and then introducing a ligand molecule monolayer sensitive to nitrite ions in a self-assembling way to obtain the nitrite ion colorimetric sensing cellulose material, wherein the nitrite ions contained in a solution can be detected by only simply soaking the nitrite ion colorimetric sensing cellulose material into an anionic water solution for certain time and observing the change of the color of the nitrite ion colorimetric sensing cellulose material by utilizing Griess reaction. The prepared nitrite ion colorimetric sensing cellulose material has excellent sensitivity and selectivity on the nitrite ions, abundant raw material sources and low price; in addition, the preparation method is simple and can realize simple, fast and economic detection of the nitrite ions.
Owner:ZHEJIANG UNIV
Metallic silver coordination polymer with three-dimensional network structure, and preparation method and application thereof
InactiveCN102516271AEasy to manufactureShort reaction timeSilver organic compoundsAnion exchangersBenzoic acidNitrite ion
The invention relates to 3,4-di(2-pyridyl)-5-(4-pyridyl)-1,2,4-triazole-silver (I) coordination polymer, and preparation and application thereof. The coordination polymer is synthesized by the following steps of: mixing an acetonitrile solution of AgNO2 and a chloroform solution of ligand L at normal temperature and normal pressure, keeping stirring for half an hour, and standing and volatizing for about one week under the shading condition to obtain a colorless blocky monocrystal product. The coordination polymer is easy to prepare, reaction time is short, the aftertreatment step is simple, and the coordination polymer is high in yield. Through the experiment, nitrite ions in the material can be selectively subjected to anion exchange reaction with fluoboric acid ions, hexafluorosilicic acid ions, nitrate radical ions and perchloric acid ions, and cannot be subjected to similar anion exchange reaction with benzoic acid ions, and acetate ions. The coordination polymer overcomes the limit of the conventional anion exchange material, an exchange process is simple and easy to realize, and the coordination polymer is expected to be actually applied in the field of ion exchange materials.
Owner:TIANJIN NORMAL UNIVERSITY
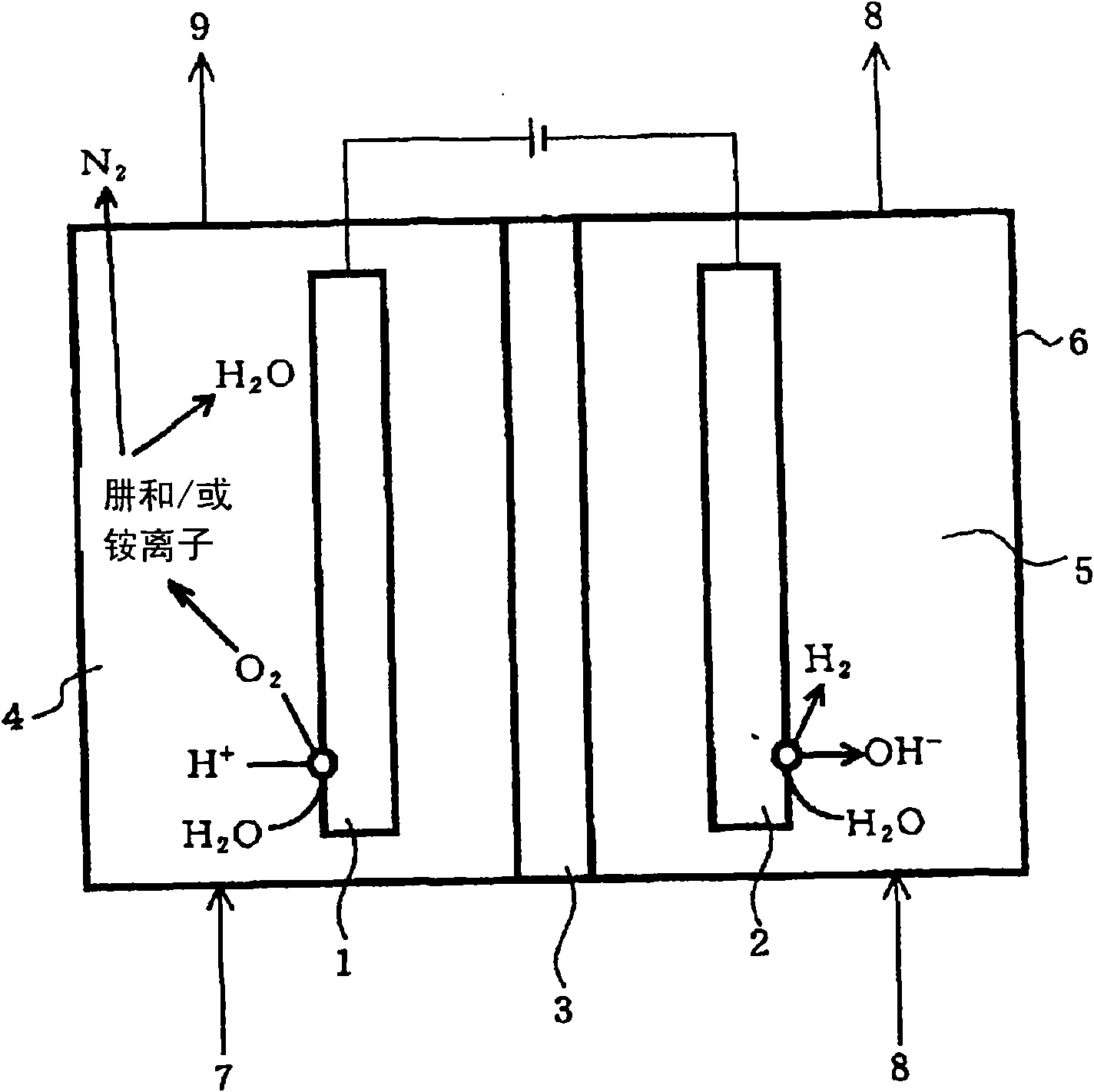
Processing method for waste water
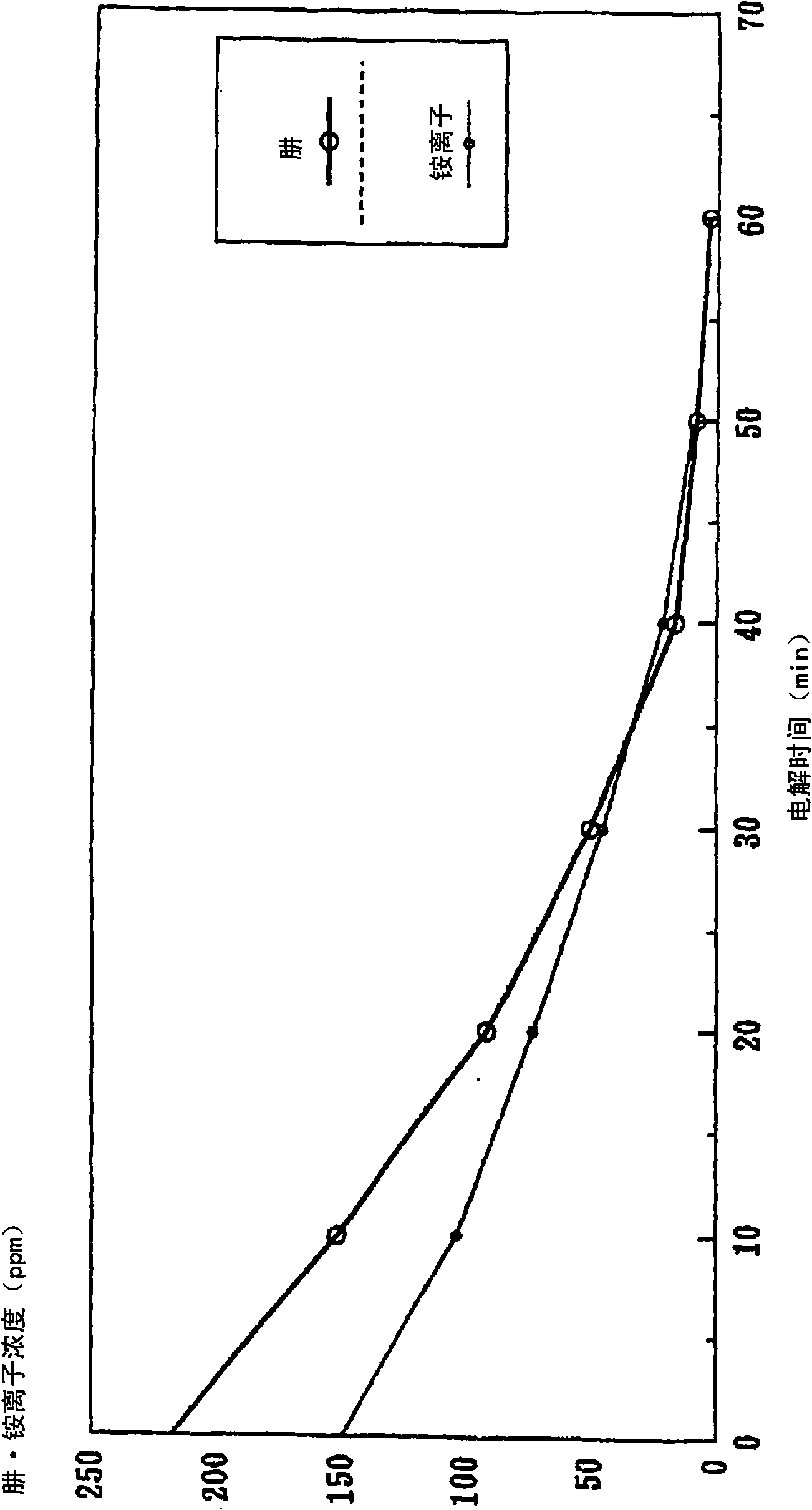
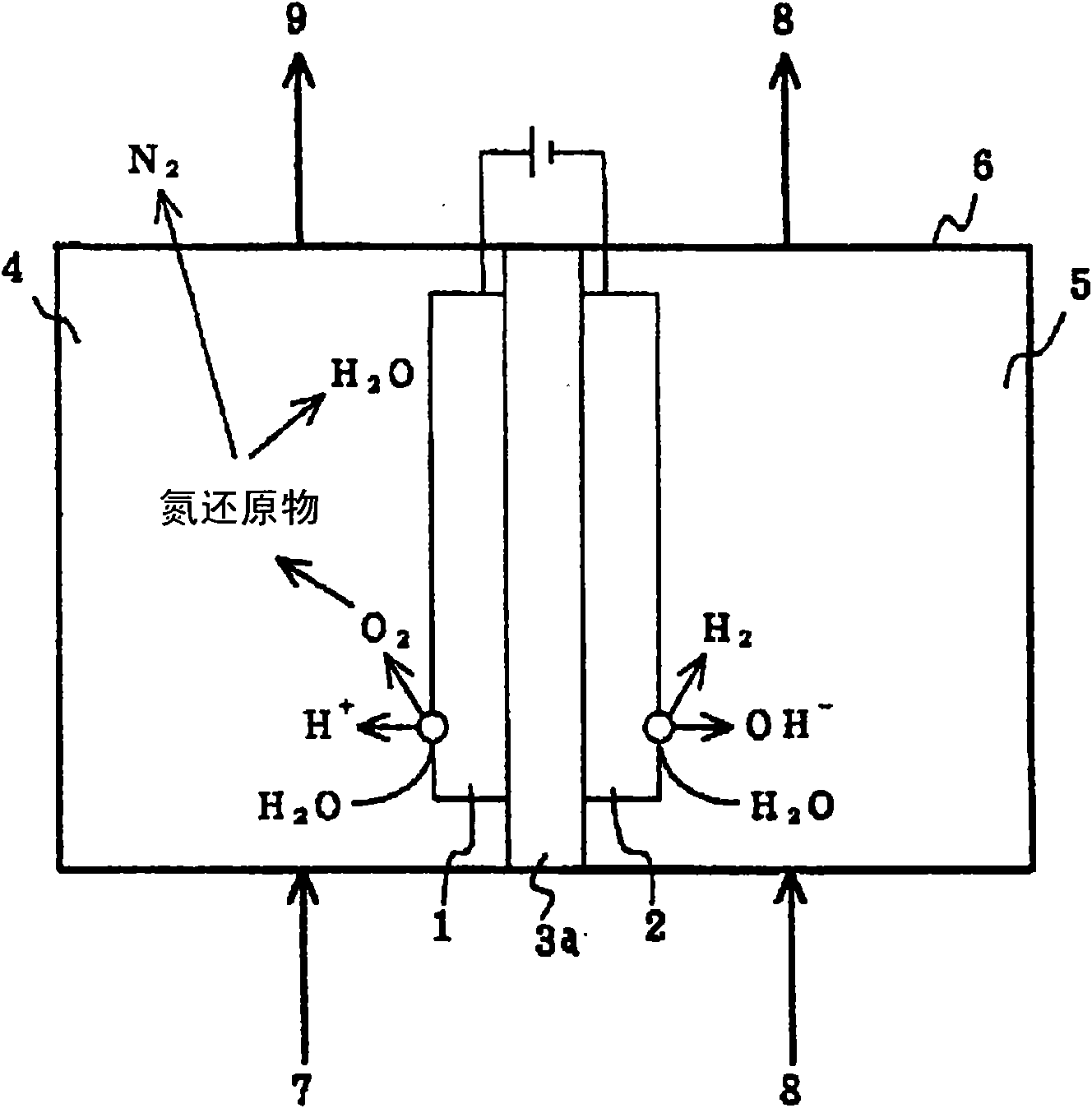
InactiveCN101643258AReduced dissolved oxygenElectrolysis componentsWater contaminantsLiquid wasteNitrite ion
The invention provides a processing method for waste water, including in the electrolytic bath, feeding pure water into the cathode room, feeding the waste water into the anode room for the electrolysis, and thereby oxidizing the reduced nitrogen compound in the waste water to nitrogen gas by oxygen generated from the water contained in the waste water; or feeding pure water into the anode room, feeding waste water into the cathode room for the electrolysis, and thereby reducing nitrogen oxide to nitrogen gas by the hydrogen generated from the water contained in the waste water; wherein a diaphragm made from alumina is provided between the anode and the cathode, at least one of the anode and the cathode is formed into a reticular structure or porous structure, moreover, the anode and the cathode are disposed in close proximity interposing the membrane therebetween. The processing method of waste water is capable of, under normal temperature and pressure, without generating the secondary waste, removing nitrogen compounds such as hydrazine, an ammonium ion, a nitrate ion, a nitrite ion, or the like as a nitrogen gas from the waste water.
Owner:KK TOSHIBA
Determination of nitrate/nitrite concentration in water by photochemical reduction
InactiveUS20110212533A1Low efficiencyEfficient reductionMaterial analysis by observing effect on chemical indicatorBiological testingNitrite ionETHYLENEDIAMINE DIHYDROCHLORIDE
Methods for determining the concentration of nitrates in several types of water samples by photochemical reduction without the use of toxic materials such as cadmium or hydrazine, and having an approximately 100% reduction efficiency are described. A water sample mixed with a buffered aqueous solution including ammonium salts and EDTA is irradiated using ultraviolet light having wavelengths effective for photochemical conversion of nitrate and nitrite ions (NOx) to detectable species. The resulting species may be quantitatively determined by diazotization using sulfanilamide followed by coupling with N-(1-napthyl)ethylenediamine dihydrochloride which produces a water-soluble azo dye having a magenta color which may be colorimetrically measured at 540 nm from which the nitrate and nitrite concentration in the sample is determined. Nitrite present in the original sample may be colorimetrically analyzed using an unphotolyzed sample and the reaction with sulfanilamide followed by coupling with N-(1-napthyl)ethylenediamine dihydrochloride. When the nitrite concentration is subtracted from the total of the nitrate and nitrite concentration, the sample nitrate may be determined.
Owner:HACH CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com