Method for detecting nitrite ions on basis of polymer carbon dot fluorescence colorimetry
A nitrite ion and carbon dot fluorescence technology, applied in the nanometer field, can solve the problems of low detection sensitivity, cumbersome synthesis route, single output signal, etc., and achieve the effect of good sensitivity and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
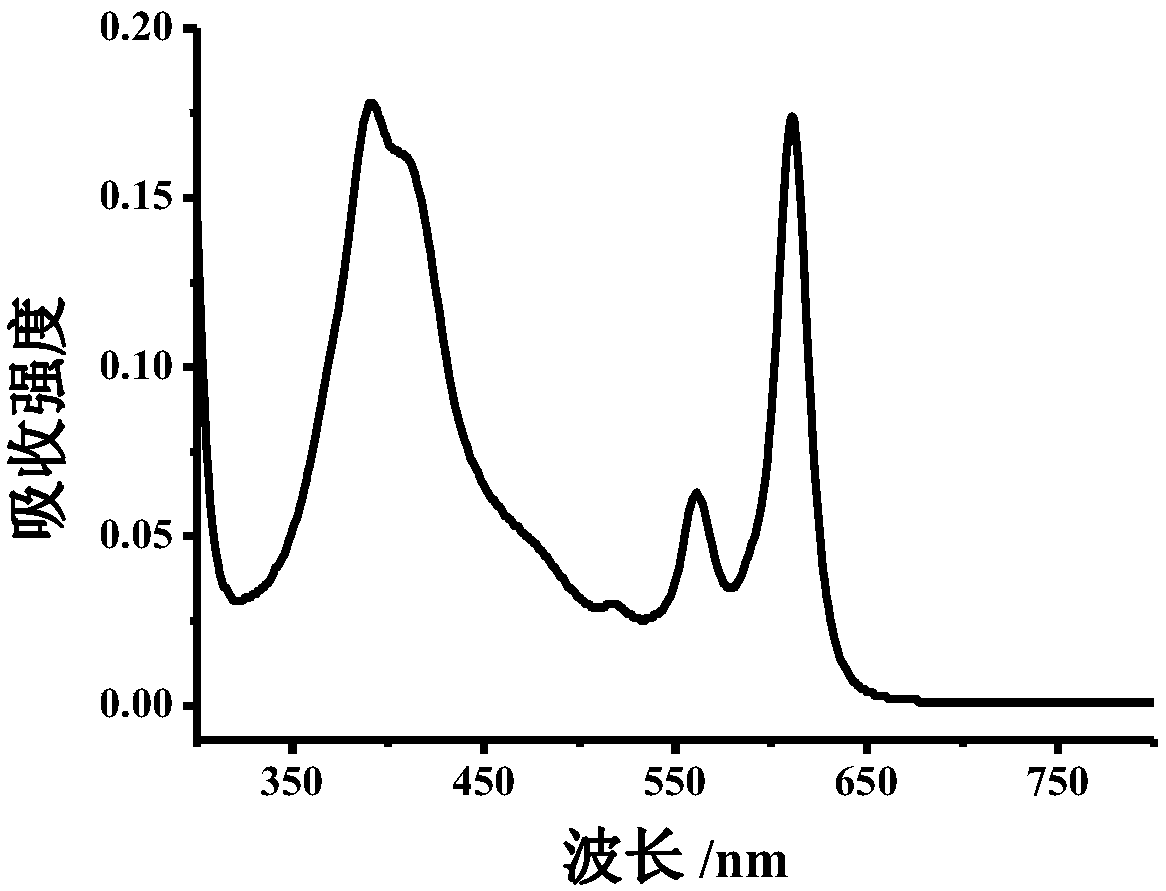
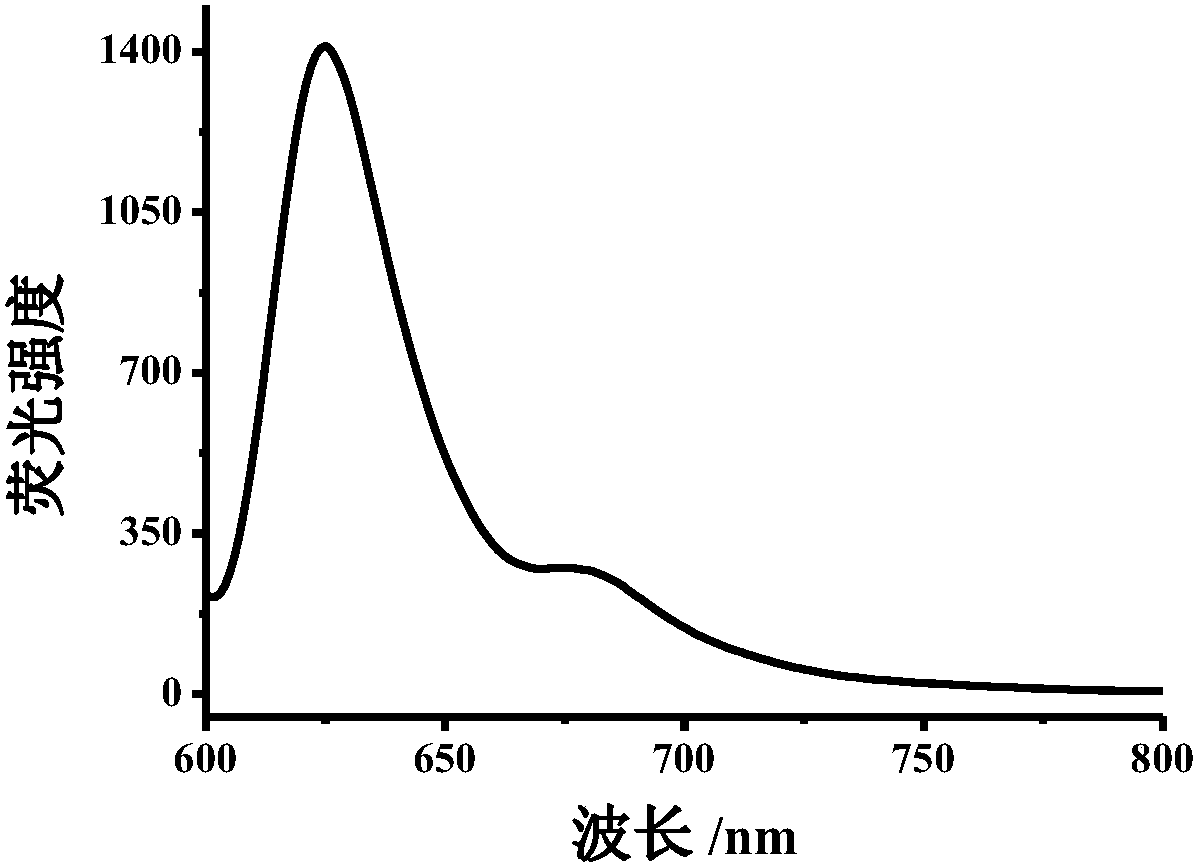
[0077] Example 1: 5 mg of dopamine hydrochloride was weighed and dissolved in 10 mL of water, and 10 mg of o-phenylenediamine and 500 μL of concentrated hydrochloric acid were added. (The molar ratio of dopamine hydrochloride to o-phenylenediamine is 1:4) Transfer the mixture to a reaction kettle (20mL) at a reaction temperature of 200°C and heat in an oven for 8h to obtain blue polymer carbon dots with a concentration of 10mg / mL . Use Tris-HCl (20mM, pH=7) buffer solution to prepare 0.4mg / mL carbon dot solution for UV-visible spectrophotometer test, record its absorption spectrum; use Tris-HCl (20mM, pH=7) buffer solution to prepare 0.2mg / mL carbon dot solution was tested by fluorescence spectrophotometer, and its emission spectrum was recorded. The test obtained absorption wavelength is 380nm, 560nm, 610nm; emission wavelength is 625nm (Ex=580nm), respectively as figure 1 and figure 2 .
Embodiment 2
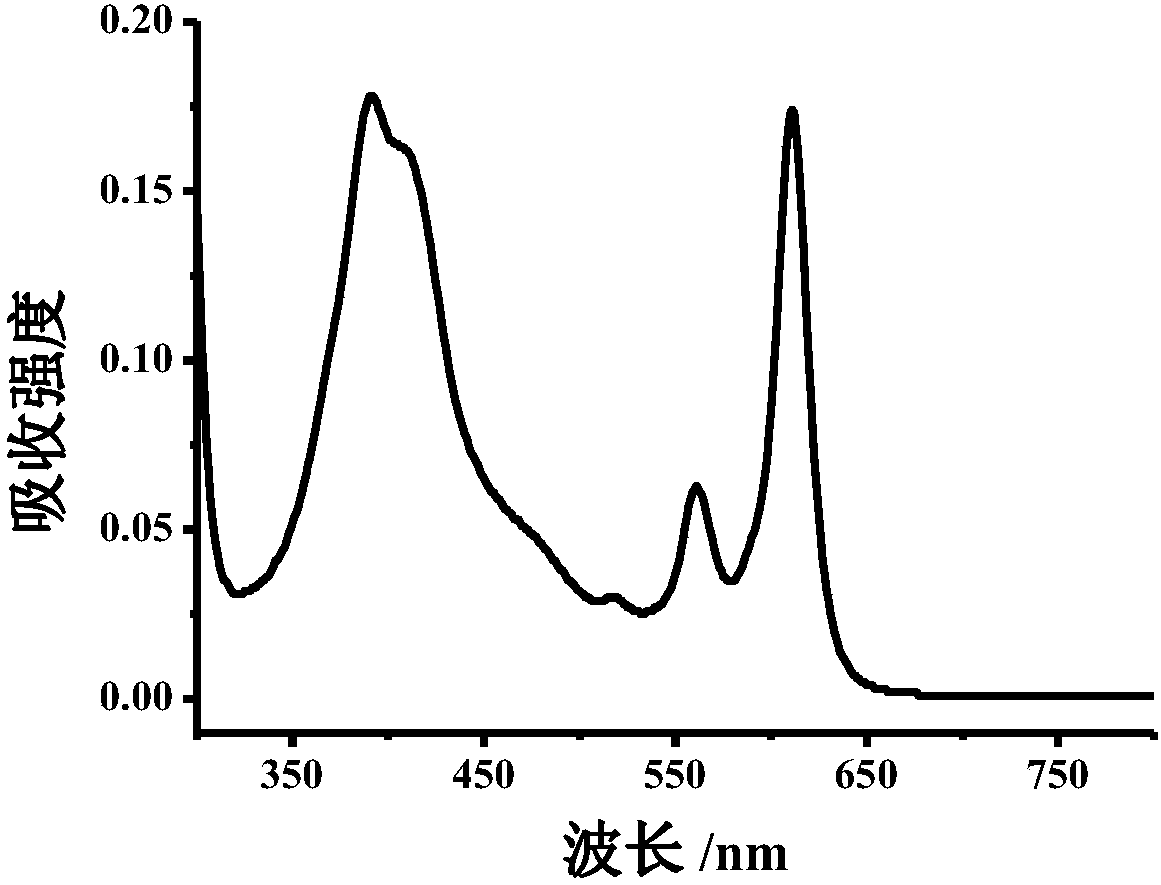
[0078] Example 2: 10 mg of dopamine hydrochloride was weighed and dissolved in 10 mL of water, and 10 mg of o-phenylenediamine and 500 μL of concentrated hydrochloric acid were added. (The molar ratio of dopamine hydrochloride to o-phenylenediamine is 1:2) The mixture was transferred to a reaction kettle (20mL) at a reaction temperature of 200°C and heated in an oven for 8h to obtain a blue solution with a concentration of 10mg / mL. Prepare 0.4mg / mL carbon dot solution with Tris-HCl (20mM, pH=7) buffer solution and carry out UV-visible spectrophotometer test, record its absorption spectrum; Tris-HCl (20mM, pH=7) buffer solution prepares 0.2mg / mL mL carbon dot solution was tested by fluorescence spectrophotometer, and its emission spectrum was recorded. The test obtained absorption wavelength is 380nm, 560nm, 610nm; emission wavelength is 625nm (Ex=580nm), respectively as image 3 and Figure 4 .
Embodiment 3
[0079] Example 3: 20 mg of dopamine hydrochloride was weighed and dissolved in 10 mL of water, and 10 mg of o-phenylenediamine and 500 μL of concentrated hydrochloric acid were added. (The molar ratio of dopamine hydrochloride to o-phenylenediamine is 1:1) The mixture was transferred to a reaction kettle (20mL) at a reaction temperature of 200°C and heated in an oven for 8h to obtain a blue solution with a concentration of 10mg / mL. Use Tris-HCl (20mM, pH=7) buffer solution to prepare 0.4mg / mL carbon dot solution for UV-visible spectrophotometer test, record its absorption spectrum; use Tris-HCl (20mM, pH=7) buffer solution to prepare 0.2mg / mL carbon dot solution was tested by fluorescence spectrophotometer, and its emission spectrum was recorded. The test obtained absorption wavelength is 380nm, 560nm, 610nm; emission wavelength is 625nm (Ex=580nm), respectively as Figure 5 and Figure 6 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com