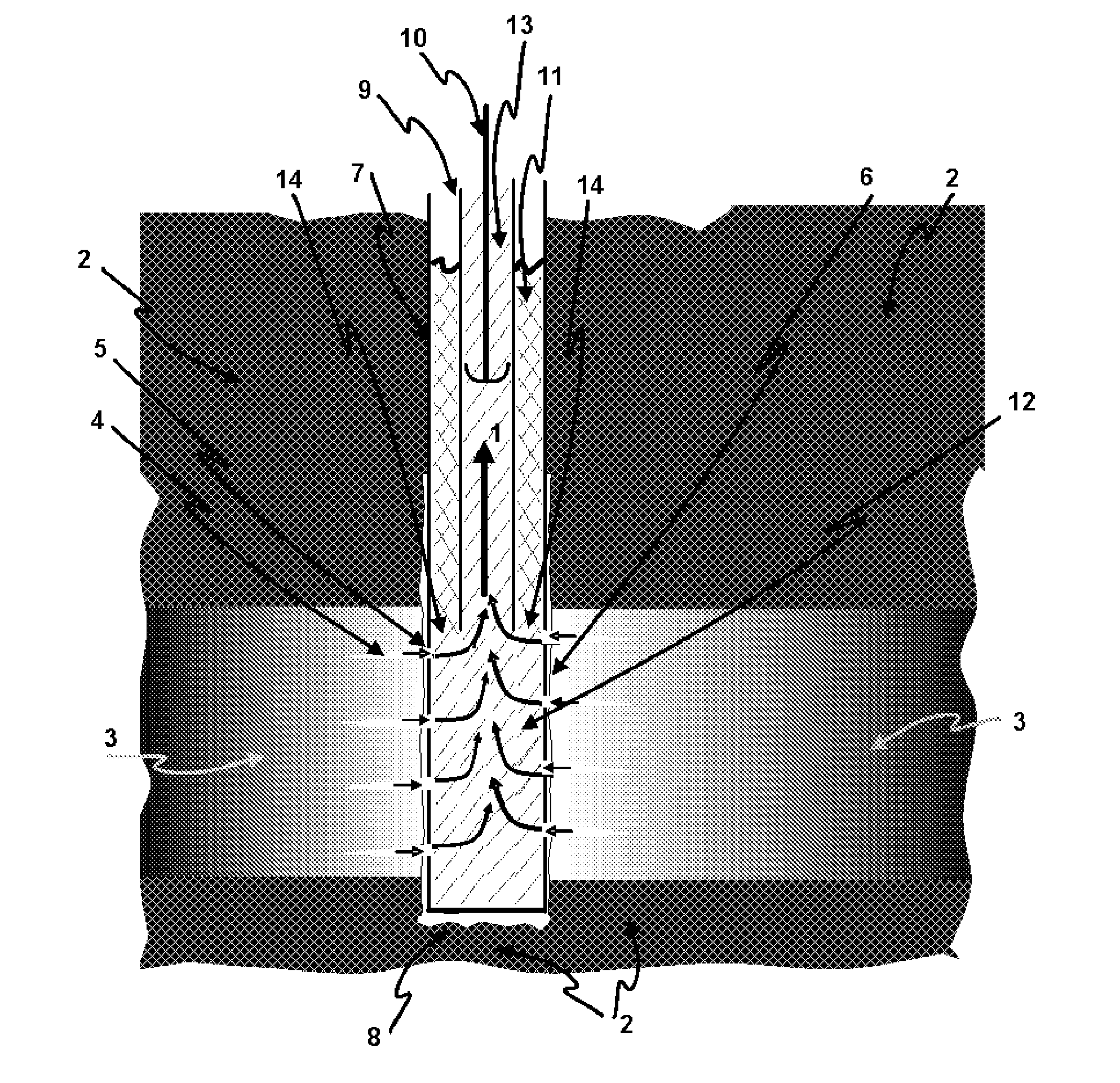
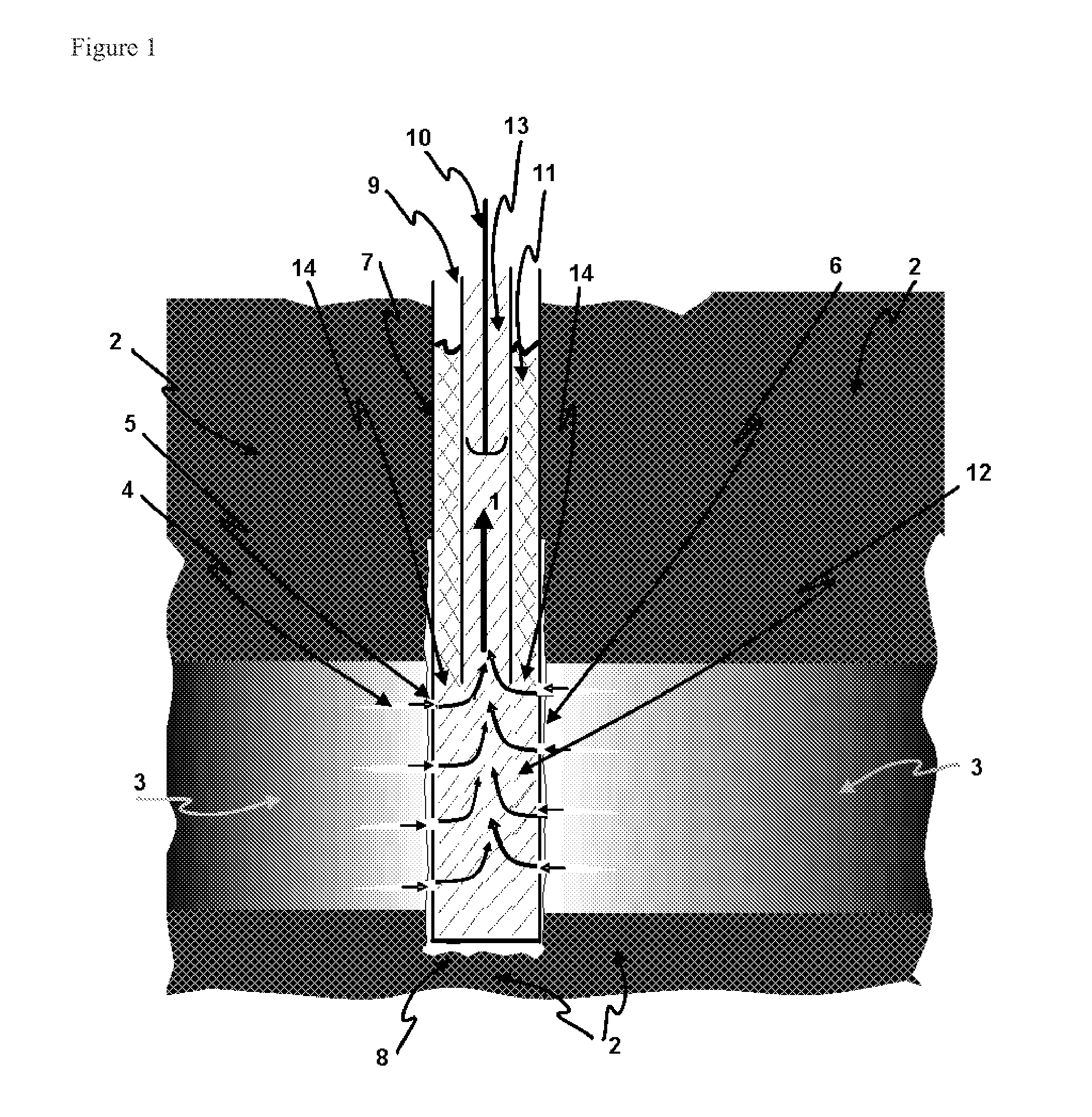
Reducing sulfide in production fluids during oil recovery
a production fluid and sulfide technology, applied in the field of oil recovery, can solve the problems of reducing the value of recovered crude oil, competition for nutrients, health and environmental hazards,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
NO2− Reactivity with Sulfide
[0061]A sodium nitrite solution was used to oxidize a sodium sulfide solution at room temperature in a closed system in order to look at the kinetics of the reaction and to prevent the volatilization of sulfide. Based on a balanced redox reaction, 1 mole of nitrite should be able to oxidize at least 0.5 mole of hydrogen sulfide. The nitrite and sulfide solutions used in the experiment were made up in artificial brine, which mimics the moderately high salinity of many oil reservoirs. The brine had the following composition: CaCl2.2H2O, 6.75 g, NaCl, 26.1 g, Na2SO4, 0.015 g, MgCl2.6H2O g, 4.45, KCl, 0.7 g plus enough water to make a total of 500 ml of brine solution. The sulfide solution in brine was approximately 15 ppm S2−. The nitrite solutions were approximately 50 ppm and 725 ppm NO2−. Two different treatments were run. In treatment 1 (Table 1) the nitrite:sulfide molar ratio was 29:1, which resulted in reaction conditions where nitrite was approximate...
example 2
NO2− Reactivity with Sulfide: Titration of Molar Ratios Needed for a Rapid Reaction
[0063]A range of nitrite:sulfide ratios were tested to determine the molar ratio needed to cause a rapid oxidation of sulfide. The nitrite and sulfide solutions used in the experiments were made up in artificial brine as described in Example 1. Experiments were performed as described in Example 1 using eight different treatments. The nitrite / sulfide molar ratios used were 2, 5, 10, 15, 20, 25, 30, and 35. Results given in Table 2 showed that the reaction rate remained slow at a ratio of 2, as seen in the previous Example, but at a ratio of 5:1 or higher the reaction occurred rapidly with sulfide becoming undetectable in 10 minutes or less. The ability to rapidly remove sulfide at lower nitrite:sulfide ratios makes the process more economical
TABLE 2Changes in sulfide concentrations with time following the additionof nitrite at NO2−:S2− ratios from 2:1 to 35:1.ppm S2−Observed ppmNO2−:S2− molarReactionad...
example 3
Prophetic
Production Well Treatment Using Continuous Addition of Nitrate / Nitrite Solution in Well Casing to Mitigate Sulfide Present in Production Fluid of Soured Wells
[0064]In this example, a producer well is continuously treated to mitigate sulfide present in the production water using a nitrate / nitrite mixture. Sulfide in the production fluids often results from well souring, following the start of water injection for secondary oil recovery. Rather than treating the injected water and all reaches of the subterranean oil reservoir, as is the normal practice to mitigate souring by sulfide formation, a process is used that treats the smaller produced water volume, making it more economical, while still sweetening the produced fluids by removing sulfide.
[0065]A nitrate / nitrite solution is produced by dissolving any inexpensive nitrate and nitrite salts, such as NaNO2 or NaNO3, in water. This solution is continuously pumped into the well casing of a production well. The production flui...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com