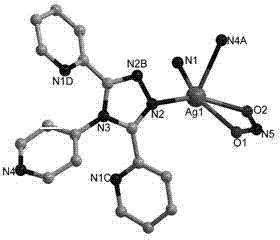
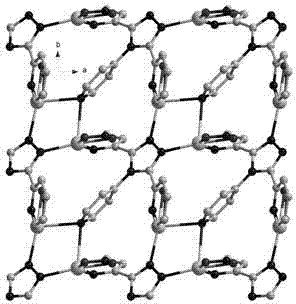
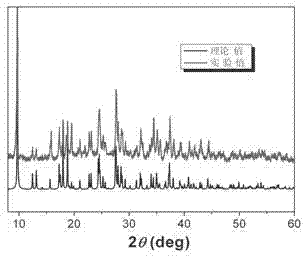
Metallic silver coordination polymer with two-dimensional lamellar structure, and preparation and application thereof
A coordination polymer, two-dimensional layered technology, applied in the field of preparation of coordination polymer materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Preparation and Discussion of Polypyridine-Bridged Organic Ligand L Containing 1,2,4-Triazole
[0062] 1. Synthesis experiment and post-processing:
[0063] Dissolve N-(4-pyridyl)-2-pyridine-thioamide (21.5 g, 0.1 mol) and 2-pyridinecarbohydrazide (13.7 g, 0.1 mol) in 200 mL of n-butanol, and heat to reflux for 36 hours After that, the heating was stopped, cooled to room temperature, filtered under reduced pressure, and washed with ethanol three times to obtain a light yellow solid. Recrystallized with ethanol to obtain a white powder product. Accurately weighed with an analytical balance, the calculated yield was 66%.
[0064] 2. Results and Discussion:
[0065] Analytical testing equipment:
[0066] Elemental analysis, C, H, N content determination using CE-440 (Leemanlabs) elemental analyzer
[0067] Infrared Spectroscopy, AVATAR-370 (Nicolet) Fourier Transform Infrared Spectrometer
[0068] Elemental Analysis: Theoretical Values (C 17 h 12 N 6 , Mr = 30...
Embodiment 2
[0071] Preparation and Discussion of Polypyridine-Bridged Organic Ligand L Containing 1,2,4-Triazole
[0072] 1. Synthesis experiment and post-processing:
[0073] Dissolve N-(4-pyridyl)-2-pyridine-thioamide (21.5 g, 0.1 mol) and 2-pyridinecarbohydrazide (17.8 g, 0.13 mol) in 200 mL of n-butanol, and heat to reflux for 40 hours After that, the heating was stopped, cooled to room temperature, filtered under reduced pressure, and washed with ethanol three times to obtain a light yellow solid. Recrystallized with ethanol to obtain a white powder product. Accurately weighed with an analytical balance, the calculated yield was 70%.
[0074] 2. Results and Discussion:
[0075] Analytical testing equipment:
[0076] Elemental analysis, C, H, N content determination using CE-440 (Leemanlabs) elemental analyzer
[0077] Infrared Spectroscopy, AVATAR-370 (Nicolet) Fourier Transform Infrared Spectrometer
[0078] Elemental Analysis: Theoretical Values (C 17 h 12 N 6 , Mr = 3...
Embodiment 3
[0081] Preparation and Discussion of Polypyridine-Bridged Organic Ligand L Containing 1,2,4-Triazole
[0082] 1. Synthesis experiment and post-processing:
[0083]Dissolve N-(4-pyridyl)-2-pyridine-thioamide (21.5 g, 0.1 mol) and 2-pyridinecarbohydrazide (20.5 g, 0.15 mol) in 200 mL of n-butanol, and heat to reflux for 38 hours After that, the heating was stopped, cooled to room temperature, filtered under reduced pressure, and washed with ethanol three times to obtain a light yellow solid. Recrystallized with ethanol to obtain a white powder product. Accurately weighed with an analytical balance, the calculated yield was 73%.
[0084] 2. Results and Discussion:
[0085] Analytical testing equipment:
[0086] Elemental analysis, C, H, N content determination using CE-440 (Leemanlabs) elemental analyzer
[0087] Infrared Spectroscopy, AVATAR-370 (Nicolet) Fourier Transform Infrared Spectrometer
[0088] Elemental Analysis: Theoretical Values (C 17 h 12 N 6 , Mr = 30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com