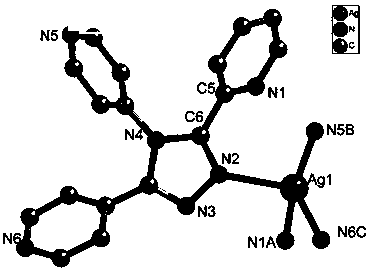
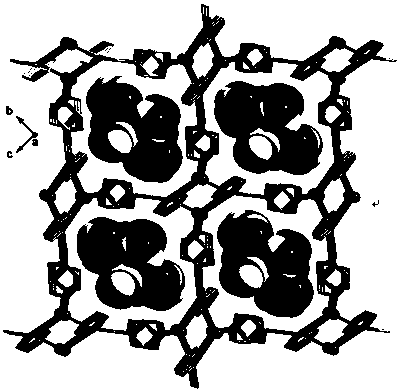
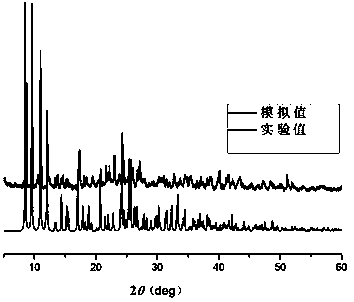
Coordination polymers with selective ion exchange properties and their applications
A coordination polymer, anion technology, applied in the application of selective anion exchange performance, 4,5-bis-3--1,2,4-triazole-silver coordination polymer preparation field, can Solve problems such as the shortage of rhenium supply, achieve the effects of short reaction time, avoid side reactions, and easy post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation and Discussion of Polypyridine-Bridged Organic Ligand (L) Containing 1,2,4-Triazole
[0039] 1. Synthesis experiment and post-processing:
[0040] Dissolve N-(4-pyridyl)-2-pyridine-thioamide (21.5 g, 0.1 mol) and 4-pyridinecarbohydrazide (13.7 g, 0.1 mol) in 200 mL of n-butanol, and heat to reflux for 36 hours After that, the heating was stopped, cooled to room temperature, filtered under reduced pressure, and washed with ethanol three times to obtain a light yellow solid. Recrystallized with ethanol to obtain a white powder product. Accurately weighed with an analytical balance, the calculated yield was 46%.
[0041] 2. Results and discussion:
[0042] Analytical testing equipment:
[0043] Elemental analysis, C, H, N content determination using CE-440 (Leemanlabs) elemental analyzer
[0044] Infrared Spectroscopy, AVATAR-370 (Nicolet) Fourier Transform Infrared Spectrometer
[0045] Elemental Analysis: Theoretical Values (C 17 h 12 N 6 , Mr = 300....
Embodiment 2
[0048] Preparation and Discussion of Polypyridine-Bridged Organic Ligand (L) Containing 1,2,4-Triazole
[0049] 1. Synthesis experiment and post-processing:
[0050] Dissolve N-(4-pyridyl)-2-pyridine-thioamide (21.5 g, 0.1 mol) and 4-pyridinecarbohydrazide (27.4 g, 0.2 mol) in 200 mL of n-butanol, and heat to reflux for 36 hours After that, the heating was stopped, cooled to room temperature, filtered under reduced pressure, and washed with ethanol three times to obtain a light yellow solid. Recrystallized with ethanol to obtain a white powder product. Accurately weighed with an analytical balance, the calculated yield was 44%.
[0051] 2. Results and discussion:
[0052] Analytical testing equipment:
[0053] Elemental analysis, C, H, N content determination using CE-440 (Leemanlabs) elemental analyzer
[0054] Infrared Spectroscopy, AVATAR-370 (Nicolet) Fourier Transform Infrared Spectrometer
[0055] Elemental Analysis: Theoretical Values (C 17 h 12 N 6 , Mr = 30...
Embodiment 3
[0058] Preparation and Discussion of Polypyridine-Bridged Organic Ligand (L) Containing 1,2,4-Triazole
[0059] 1. Synthesis experiment and post-processing:
[0060] Dissolve N-(4-pyridyl)-2-pyridine-thioamide (21.5 g, 0.1 mol) and 4-pyridinecarbohydrazide (20.6 g, 0.15 mol) in 200 mL of n-butanol, and heat to reflux for 40 hours After that, the heating was stopped, cooled to room temperature, filtered under reduced pressure, and washed with ethanol three times to obtain a light yellow solid. Recrystallized with ethanol to obtain a white powder product. Accurately weighed with an analytical balance, the calculated yield was 42%.
[0061] 2. Results and discussion:
[0062] Analytical testing equipment:
[0063] Elemental analysis, C, H, N content determination using CE-440 (Leemanlabs) elemental analyzer
[0064] Infrared Spectroscopy, AVATAR-370 (Nicolet) Fourier Transform Infrared Spectrometer
[0065] Elemental Analysis: Theoretical Values (C 17 h 12 N 6 , Mr = 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com