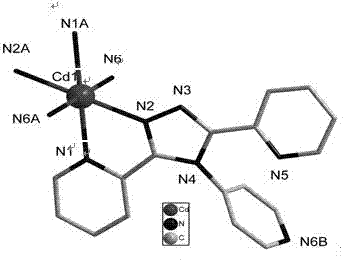
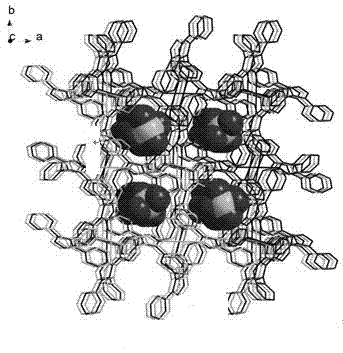
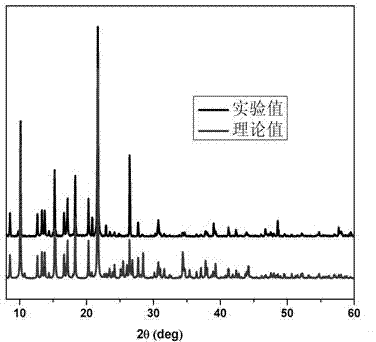
Coordination polymer with three-dimensional open framework structure and its preparation and application
A technology of coordination polymers and skeleton structures, applied in cadmium organic compounds, organic chemistry, chemical/physical processes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Preparation and Discussion of Polypyridine Bridged Organic Ligand L Based on 1,2,4-Triazole
[0064] 1. Synthesis experiment and post-processing:
[0065] Dissolve N-(4-pyridyl)-2-pyridine-thioamide (21.5 g, 0.1 mol) and 2-pyridinecarbohydrazide (13.7 g, 0.1 mol) in 200 mL of n-butanol, and heat to reflux for 36 hours After that, the heating was stopped, cooled to room temperature, and filtered under reduced pressure. The obtained light yellow solid was washed three times with ethanol, and then recrystallized with ethanol to obtain a white powder product. Accurately weighed with an analytical balance, the calculated yield was 66%.
[0066] 2. Results and Discussion:
[0067] Analytical testing equipment:
[0068] Elemental analysis, C, H, N content determination using CE-440 (Leemanlabs) elemental analyzer
[0069] Infrared Spectroscopy, AVATAR-370 (Nicolet) Fourier Transform Infrared Spectrometer
[0070] Elemental Analysis: Theoretical Values (C 17 h 12 N ...
Embodiment 2
[0073] Preparation and Discussion of Polypyridine Bridged Organic Ligand L Based on 1,2,4-Triazole
[0074] 1. Synthesis experiment and post-processing:
[0075] Dissolve N-(4-pyridyl)-2-pyridine-thioamide (21.5 g, 0.1 mol) and 2-pyridinecarbohydrazide (17.8 g, 0.13 mol) in 200 mL of n-butanol, and heat to reflux for 40 hours After that, the heating was stopped, cooled to room temperature, and filtered under reduced pressure. The obtained light yellow solid was washed three times with ethanol, and then recrystallized with ethanol to obtain a white powder product. Accurately weighed with an analytical balance, the calculated yield was 70%.
[0076] 2. Results and Discussion:
[0077] Analytical testing equipment:
[0078] Elemental analysis, C, H, N content determination using CE-440 (Leemanlabs) elemental analyzer
[0079] Infrared Spectroscopy, AVATAR-370 (Nicolet) Fourier Transform Infrared Spectrometer
[0080] Elemental Analysis: Theoretical Values (C 17 h 12 N 6...
Embodiment 3
[0083] Preparation and Discussion of Polypyridine Bridged Organic Ligand L Based on 1,2,4-Triazole
[0084] 1. Synthesis experiment and post-processing:
[0085] Dissolve N-(4-pyridyl)-2-pyridine-thioamide (21.5 g, 0.1 mol) and 2-pyridinecarbohydrazide (20.5 g, 0.15 mol) in 200 mL of n-butanol, and heat to reflux for 38 hours After that, the heating was stopped, cooled to room temperature, and filtered under reduced pressure. The obtained light yellow solid was washed three times with ethanol, and then recrystallized with ethanol to obtain a white powder product. Accurately weighed with an analytical balance, the calculated yield was 73%.
[0086] 2. Results and Discussion:
[0087] Analytical testing equipment:
[0088] Elemental analysis, C, H, N content determination using CE-440 (Leemanlabs) elemental analyzer
[0089] Infrared Spectroscopy, AVATAR-370 (Nicolet) Fourier Transform Infrared Spectrometer
[0090] Elemental Analysis: Theoretical Values (C 17 h 12 N ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com