Metallic silver coordination polymer with three-dimensional network structure, and preparation method and application thereof
A technology of coordination polymer and network structure, applied in the field of structure and performance, synthesis of new inorganic-organic hybrid materials, achieving high yield, convenient operation, and avoiding side reactions
Inactive Publication Date: 2014-05-28
TIANJIN NORMAL UNIVERSITY
View PDF1 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, currently known coordination polymers with anion exchange function often show certain limitations
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
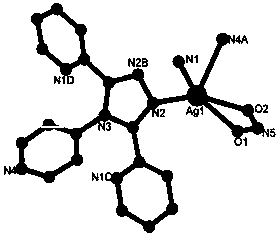
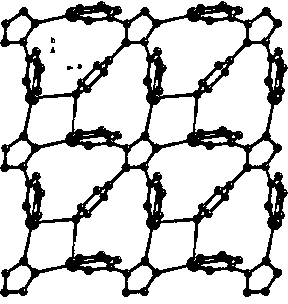
The invention relates to 3,4-di(2-pyridyl)-5-(4-pyridyl)-1,2,4-triazole-silver (I) coordination polymer, and preparation and application thereof. The coordination polymer is synthesized by the following steps of: mixing an acetonitrile solution of AgNO2 and a chloroform solution of ligand L at normal temperature and normal pressure, keeping stirring for half an hour, and standing and volatizing for about one week under the shading condition to obtain a colorless blocky monocrystal product. The coordination polymer is easy to prepare, reaction time is short, the aftertreatment step is simple, and the coordination polymer is high in yield. Through the experiment, nitrite ions in the material can be selectively subjected to anion exchange reaction with fluoboric acid ions, hexafluorosilicic acid ions, nitrate radical ions and perchloric acid ions, and cannot be subjected to similar anion exchange reaction with benzoic acid ions, and acetate ions. The coordination polymer overcomes the limit of the conventional anion exchange material, an exchange process is simple and easy to realize, and the coordination polymer is expected to be actually applied in the field of ion exchange materials.
Description
This application was supported by Tianjin Natural Science Foundation (No.10JCZDJC21800); National Natural Science Foundation of China (21101116). Technical field The invention belongs to the technical field of synthesis, structure and performance of novel inorganic-organic hybrid materials, and particularly relates to the preparation of a coordination polymer material with a three-dimensional network structure and its application in selective anion exchange. Background technique In recent years, due to the novel and interesting crystal structure of coordination polymers and the huge application potential in the fields of optics, magnetism, adsorption, ion exchange and catalysis, the use of crystal engineering strategies to design and synthesize coordination polymers has attracted widespread attention (SL James, Chem. Soc. Rev. 2003, 32, 276-288; B. Moulton, MJ Zaworotko, Chem. Rev. 2001, 101, 1629-1658; L. Carlucci, G. Ciani, DM Proserpio, Coord. Chem. Rev. 2003, 246, 247-289;...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07F1/10C07D401/14B01J41/12B01J41/13
Inventor 李程鹏阎艳杜淼陈静
Owner TIANJIN NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com