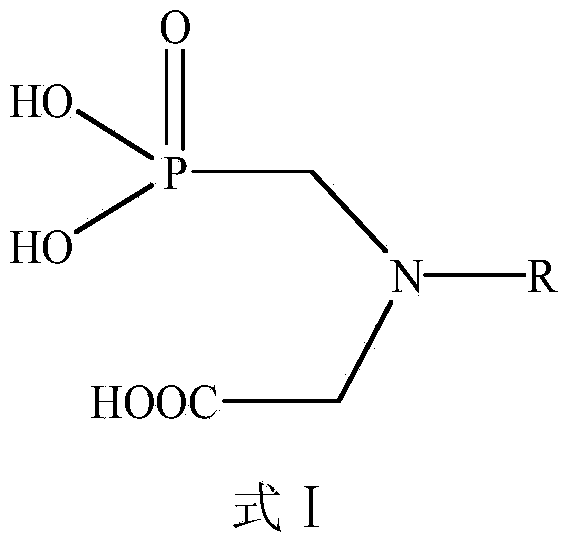
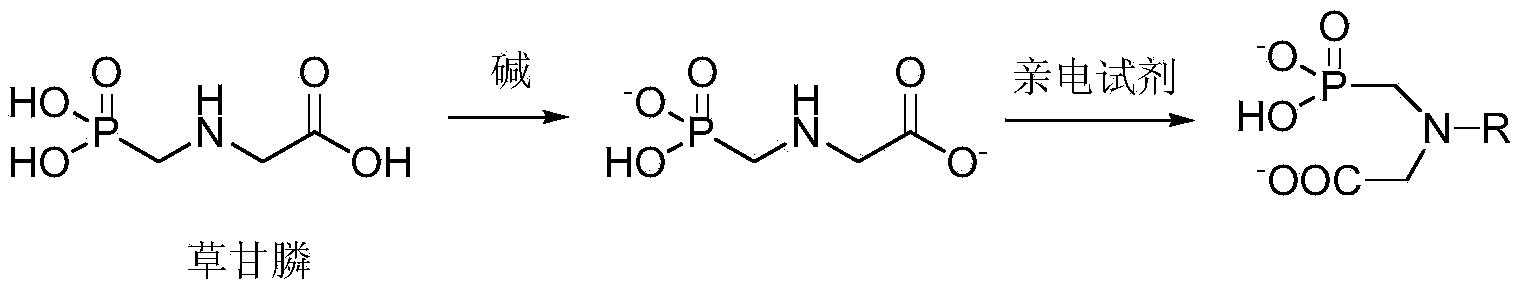
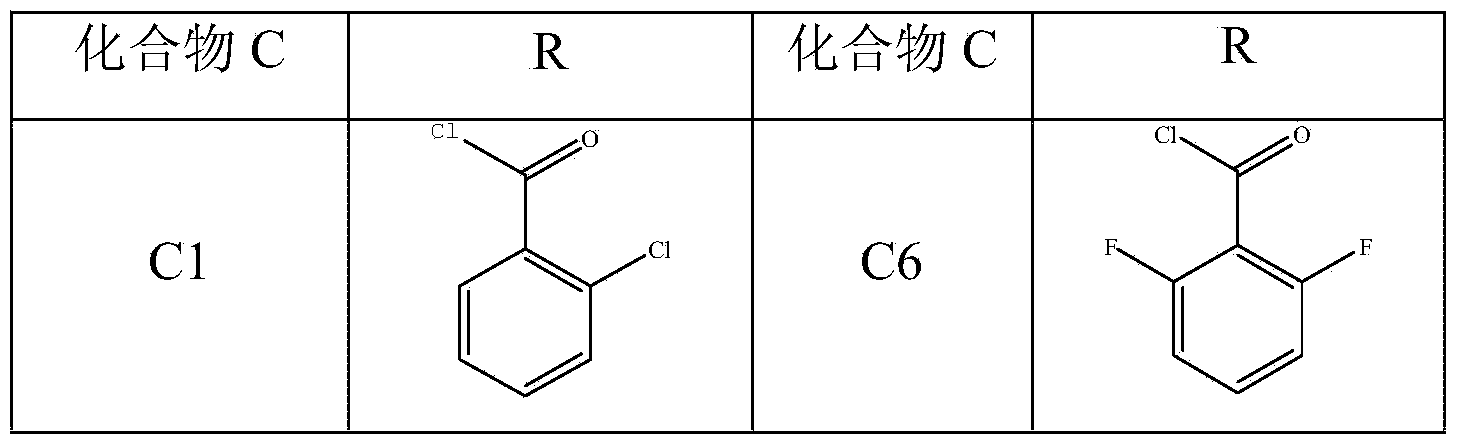
Aromatic amide compound comprising phosphoryl amino acid structure, preparation method of compound and application of compound taken as weed killer
A phosphoryl amino acid and aromatic amide technology, applied in the field of pesticide chemistry, can solve the problems of poor killing effect on broad-leaved weeds, serious resistance problems, slow drug response, etc., and achieve good herbicidal activity, rapid action, and high activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1. Preparation of compound C1 (N-(2-chloro-benzoyl)-N-phosphorylmethyl-glycine)
[0036] Add 0.01mol of glyphosate and 50ml of water to a 250ml four-necked bottle equipped with magnetic stirring, cooling, thermometer, and nitrogen protection, drop in 40% aqueous sodium hydroxide solution until the total amount of alkali is 0.04mol, cool and stir until The temperature of the system is 5°C, add 0.012mol of 2-chlorobenzoyl chloride tetrahydrofuran solution dropwise to the four-neck flask, after the dropwise addition, react at room temperature for 5 hours, stop stirring, separate the liquid to take the oil layer, and recrystallize methanol after rotary evaporation and precipitation , to obtain compound C1 with a yield of 65.5% and a purity of 99.6%.
[0037] The carbon spectrum data of N-(2-chloro-benzoyl)-N-phosphorylmethyl-glycine 13 C NMR (CDCl 3 )δ ppm =48.7,55.1,124.4,128.7,131.1,133.9,135.3,136.9,161.4,176.6.
[0038] After characterization, it was the tar...
Embodiment 2
[0039] Example 2, preparation of compound C2 (N-(4-chloro-benzoyl)-N-phosphorylmethyl-glycine)
[0040] Add 0.01 mol of glyphosate and 50 ml of dimethylformamide to a 250 ml four-neck flask equipped with magnetic stirring, cooling, thermometer, and nitrogen protection, drop 30% aqueous solution of sodium carbonate until the total amount of alkali is 0.03 mol, cool Stir until the system temperature is 10°C; add 0.015mol 4-chlorobenzoyl chloride dimethylformamide solution dropwise to the four-neck flask, after the dropwise addition, react at room temperature for 6 hours, distill under reduced pressure to remove the solvent, and dissolve in dichloromethane , washed with water, separated into the oil layer, recrystallized from methanol after rotary evaporation and desolventization, and obtained compound C2 with a yield of 69.4% and a purity of 99.8%.
[0041] The carbon spectrum data of N-(4-chloro-benzoyl)-N-phosphorylmethyl-glycine 13 C NMR (CDCl 3 )δ ppm =47.5,52.4,127.2,128...
Embodiment 3
[0043] Example 3. Preparation of compound C3 (N-(2,6-dichloro-benzoyl)-N-phosphorylmethyl-glycine)
[0044] Add 0.01mol of glyphosate and 50ml of dimethyl sulfoxide to a 250ml four-necked flask equipped with magnetic stirring, cooling, thermometer, and nitrogen protection, drop in 0.05mol of triethylamine, cool and stir until the system temperature is -5°C ; Add 0.011mol dimethyl sulfoxide solution of 2,6-dichlorobenzoyl chloride dropwise to the four-neck flask. After the dropwise addition, react at room temperature for 4 hours, and after desolvation by distillation under reduced pressure, dissolve dichloromethane and wash with water. The oil layer was obtained by liquid separation, methanol was recrystallized after rotary evaporation and desolventization, and compound C3 was obtained with a yield of 66.2% and a purity of 99.3%.
[0045] The carbon spectrum data of N-(2,6-dichloro-benzoyl)-N-phosphorylmethyl-glycine 13 C NMR (CDCl 3 )δ ppm =47.7,55.1,126.4,126.4,132.3,136.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com