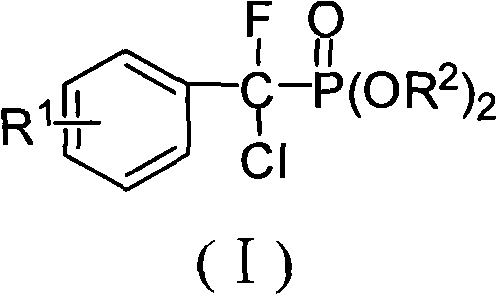
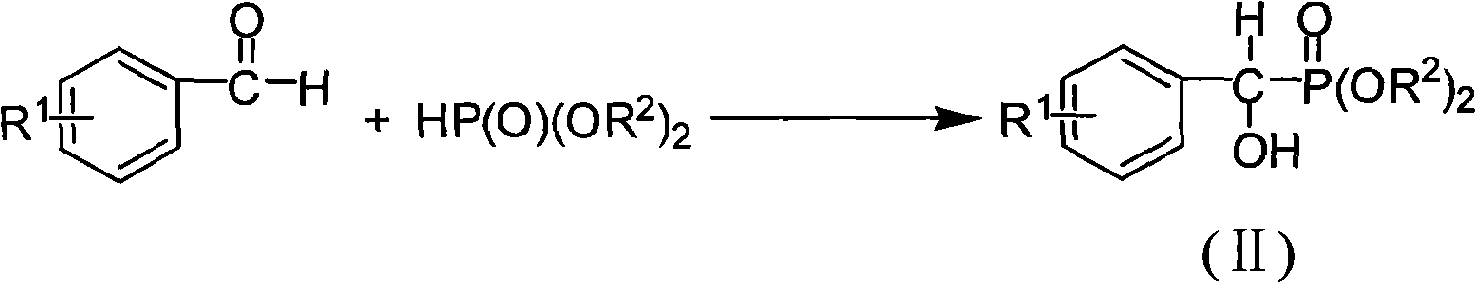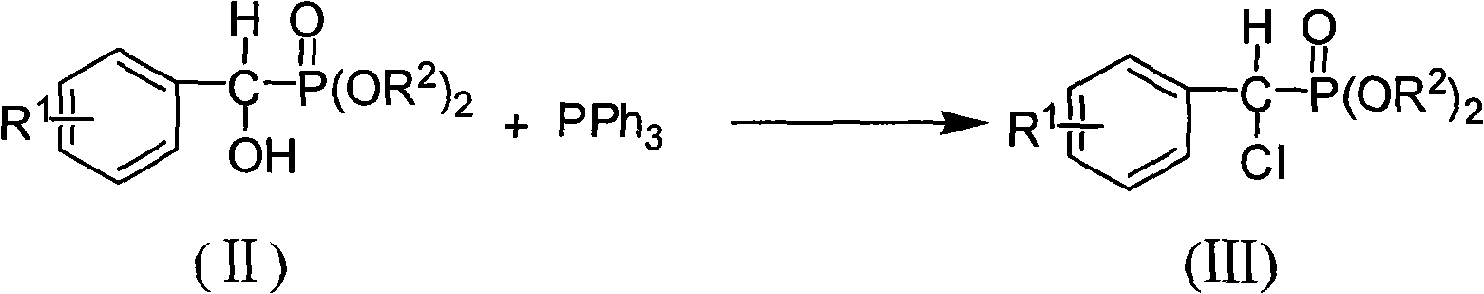Alpha, alpha-fluorine chlorine fragrant methyl phosphonate and method for preparing the same
A technology of fluorochloroarylmethylphosphonate and diethyl benzylphosphonate is applied in the field of organic compounds, and can solve the problems of unreported preparation methods, and achieve the effects of high yield and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, α, the preparation of α-fluorochlorobenzyl phosphonic acid diethyl ester
[0038] a, the preparation of diethyl α-hydroxybenzylphosphonate
[0039] Weigh 2.50g (23.56mmol, 1.0eq.) of benzaldehyde, add 10mL of dichloromethane to dissolve, and prepare the benzaldehyde solution, and set aside; weigh 2.40g (35.34mmol, 1.5eq.) of sodium ethoxide, add dichloromethane 10mL was dissolved, and then 2.44mL (18.85mmol, 0.8eq.) of diethyl phosphinate was added at -35°C under the protection of argon. After stirring for 30 minutes, the above-mentioned benzaldehyde solution was added, and the reaction was continued. Monitor the reaction process with thin-layer chromatography (TLC) method, add concentration after 3 hours and be the hydrochloric acid termination reaction of 0.1mol / L, reaction solution is extracted with ethyl acetate, get organic layer, dry with anhydrous magnesium sulfate, suction filtration, filtrate The solvent was recovered by distillation under reduce...
Embodiment 2
[0047] Embodiment 2, α, the preparation of α-fluorochloro-(4-fluoro)benzylphosphonic acid diethyl ester
[0048] a, the preparation of α-hydroxyl-(4-fluoro)benzylphosphonic acid diethyl ester
[0049] Weigh 2.00g (16.12mmol, 1.0eq.) of p-fluorobenzaldehyde, add 5mL of dichloromethane to dissolve, and prepare a p-fluorobenzaldehyde solution, and set aside; weigh 1.86g (27.40mmol, 1.7eq.) of sodium ethoxide, Add 10 mL of dichloromethane to dissolve, then add 2.19 mL of diethyl phosphinate (16.92 mmol, 1.05 eq.) at a temperature of -35 °C under the protection of argon, stir and react for 30 minutes, then add the above-mentioned p-fluorobenzaldehyde solution, continue to stir the reaction, monitor the reaction process with the TLC method, add concentration after 3 hours and be the hydrochloric acid termination reaction of 0.1mol / L, the reaction solution is extracted with ethyl acetate, get the organic layer, dry with anhydrous magnesium sulfate, suction filtration, The filtrate w...
Embodiment 3
[0057] Embodiment 3, α, the preparation of α-fluorochloro-(4-chloro) benzyl phosphonic acid diethyl ester
[0058] The preparation of a, α-hydroxyl-(4-chloro) benzyl phosphonic acid diethyl ester
[0059] Referring to Example 2, using p-chlorobenzaldehyde as a starting material for preparation, the product is identified as α-hydroxyl-(4-chloro)benzylphosphonic acid diethyl ester by NMR:
[0060] 1 H NMR (300MHz, CDCl 3 ): δ=1.22~1.30(m, 6H), 3.96~4.13(m, 4H), 5.00(d, 2 J PH =10.7Hz, 1H), 7.34(d, J=8.3Hz, 2H), 7.43(d, J=8.5Hz, 2H)ppm; 13 CNMR (75MHz, CDCl 3 ): δ=16.3(d, 3 J PC =5.5Hz), 63.0(d, 2 J PC =7.4Hz), 63.4(d, 2 J PC =7.0Hz), 69.9(d, 1 J PC =159.6Hz), 128.3, 128.4, 133.6, 135.5ppm.
[0061] B, the preparation of α-chloro-(4-chloro) benzyl phosphonic acid diethyl ester
[0062] Prepared with reference to Example 2, the product yield is 87%, identified as α-chloro-(4-chloro)benzylphosphonic acid diethyl ester by NMR:
[0063] 1 H NMR (300MHz, CDCl 3 ): δ=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com