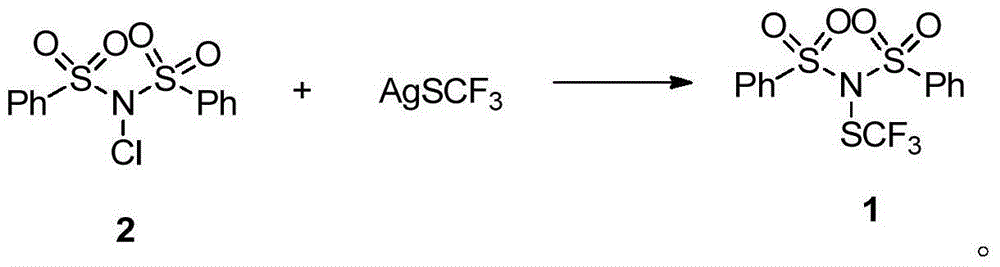Trifluoromethylthiolation reagent, and preparation method and application thereof
A technology of trifluoromethylthio group and reagent, applied in the preparation of thioether, organic chemistry, etc., can solve the problems of high toxicity of trifluoromethylthio group reagent, low reactivity, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
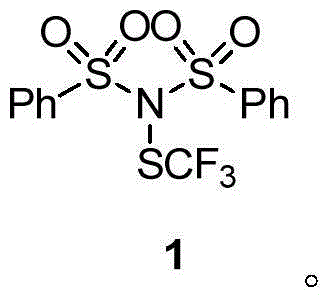
[0056] The preparation of embodiment 1 trifluoromethylthiolation reagent 1
[0057]
[0058] Under the condition of Ar gas protection, compound 2 (2.0g, 6mmol), AgSCF 3 (1.76g, 8.4mmol) was added to a 50mL dry Schlenk bottle equipped with a stir bar, and 20mL of redistilled dichloromethane was added, and stirred at room temperature (about 25°C) in the dark for 2 hours. After the reaction was finished, the AgCl precipitate was filtered, the sample was mixed with silica gel, and the white solid trifluoromethylthiolation reagent 1 was obtained by column chromatography. (1.50g, yield 65%, purity greater than 98% identified by hydrogen spectrum.).
[0059] N-benzenesulfonyl-N-trifluoromethylthiobenzenesulfonamide (N-(Phenylsulfonyl)-N-((trifluoromethyl)thio)benzenesulfonamide): yield 65%, white solid. 1 H NMR (400MHz, CDCl 3 ,293K,TMS)δ8.05(d,J=8.1Hz,4H),7.71(t,J=7.4Hz,2H),7.58(t,J=7.7Hz,4H); 19 F NMR (376MHz, CDCl 3 )δ-48.20(s,3F); 13 C NMR (126MHz, CDCl 3 , 293K, TMS) δ...
Embodiment 2
[0062] Naphthalene was used as an aromatic hydrocarbon substrate and reacted for 24 hours. The screening conditions for reaction temperature, concentration, reagent equivalent, and acid dosage are shown in Table 1. x represents the charging capacity of substrate naphthalene; y represents the charging capacity of the trifluoromethylthiolation reagent shown in formula 1; z represents the charging capacity of acid; a Indicates that the molar volume ratio of trifluoromethylthiolation reagent 1 to solvent is 0.1mol / L; 19 F NMR yield is internal standard with trifluorotoluene; b Indicates that the molar volume ratio of trifluoromethylthiolation reagent 1 to solvent is 0.5mol / L; NR indicates that no reaction product was detected.
[0063]
[0064] Table 1 Screening table of reaction conditions
[0065]
[0066]
Embodiment 3
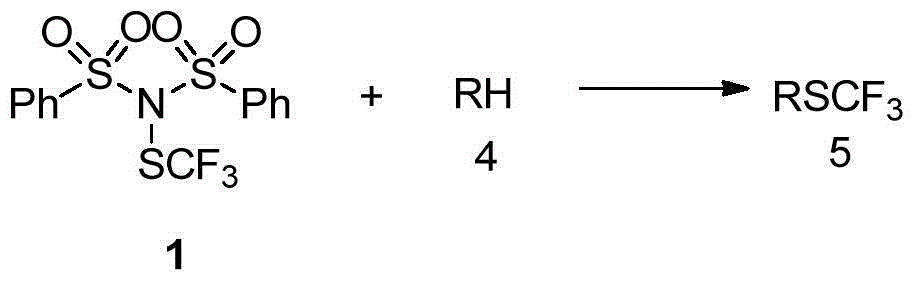
[0067] Embodiment 3 The application of the trifluoromethylthiolation reagent shown in formula 1, the specific operating conditions are as follows:
[0068] Indole substrate reaction operation steps: under the condition of argon (Ar) protection, the indole substrate (0.3mmol), the trifluoromethylthiolation reagent 1 (0.36mmol) shown in formula 1 is added to already Add 1.5 mL of redistilled dichloromethane to a 25 mL sealed tube with a stirring bar, and react for 24 hours at room temperature (about 20°C to 25°C). TLC was followed until the reaction was completed, the sample was mixed with silica gel, and the corresponding products (a1-a7) were separated by flash column chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com