Green preparation technique of 1,4-diiodo-benzene
A technology of diiodobenzene and p-iodoaniline, which is applied in the field of green preparation technology of 1,4-diiodobenzene, can solve the problems of large amount of hydroiodic acid, insufficient reaction, high reaction cost, etc., and achieve high product purity and reduce Production cost, simplification of operation engineering effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
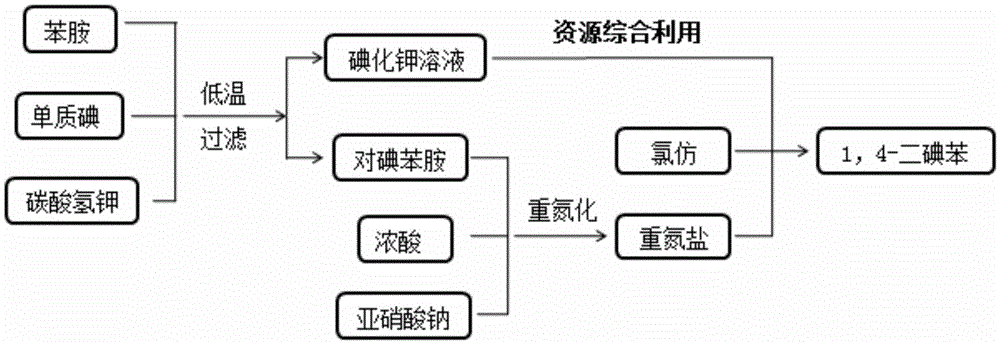
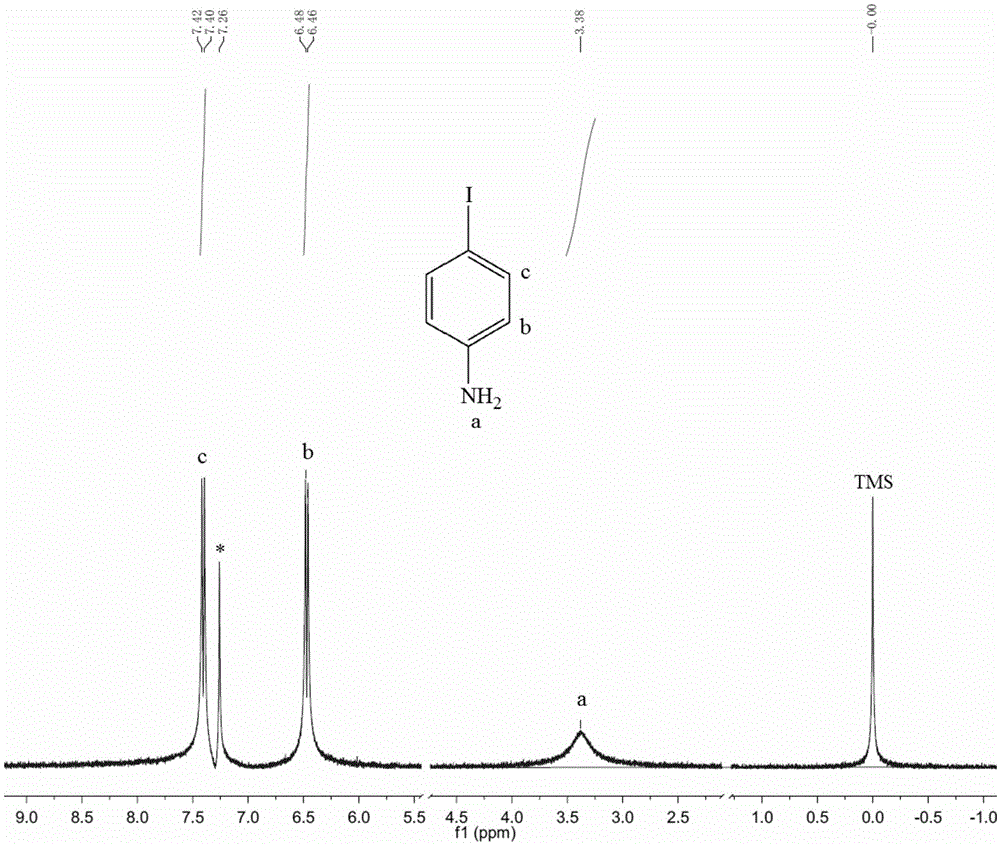
[0033] Such as figure 1 Process flow: Add 9.3g of aniline (0.1mol) to 110ml of 10% (w / w) potassium bicarbonate solution, then add crushed iodine to aniline molar ratio of 1.05:1 within 15 to 20 minutes 26.7g of elemental iodine was subjected to electrophilic substitution reaction at 5-10°C for 4 hours, and filtered after the reaction was completed. The liquid is the potassium iodide solution generated by the reaction, which is directly used for the iodine displacement reaction. After the solid was dried, 21.0 g of p-iodoaniline was obtained with a yield of 95.8%. Such as figure 2 , proton nuclear magnetic resonance spectrum analysis result: 1 HNMR (CDCl3, 270MHz), 7.40(d, 2H), 6.47(d, 2H), 3.38(s, 2H), nuclear magnetic resonance test showed that it was the target product.
[0034] Add 21.0g of p-iodoaniline (0.096mol) into 400 ml of water, control the reaction temperature below 0°C, add 90 ml of 36.5% (w / w) concentrated hydrochloric acid with a molar ratio of hydrochloric...
Embodiment 2
[0037] Add 13.95 g of aniline (0.15 mol) to 110 ml of 15% (w / w) potassium bicarbonate solution, then add 40.0 g of crushed iodine to aniline with a molar ratio of 1.05:1 in batches within 15 to 20 minutes Elemental iodine, carry out electrophilic substitution reaction at 5-10°C for 4 hours, and filter after the reaction is completed. The liquid is the potassium iodide solution generated by the reaction, which is directly used for the iodine displacement reaction. After the solid was dried, 31.1 g of p-iodoaniline crude product was obtained, with a yield of 94.5%, which was shown to be the target product by nuclear magnetic resonance.
[0038] 31.1g p-iodoaniline (0.142mol) is added to 600 milliliters of water, control reaction temperature is less than 0 ℃, add sulfuric acid and p-iodoaniline mol ratio is 5.25: 1 the concentrated sulfuric acid of 40.5 milliliters 98% (w / w), quick Cool to -10°C to precipitate ultra-fine white crystals, then add dropwise 50 ml of 20% (w / w) sodiu...
Embodiment 3
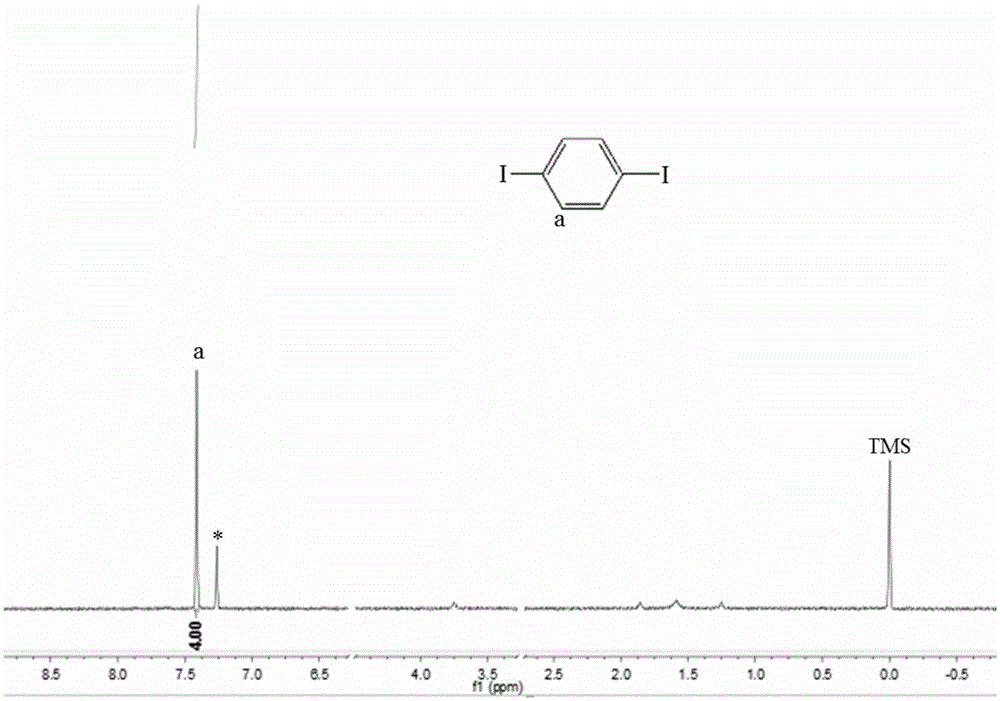
[0041] 1.86kg aniline (20mol) was added to 20 liters of 10% (w / w) potassium bicarbonate solution, then within 40 minutes, 5.34kg elemental iodine ( 21mol), the electrophilic substitution reaction was carried out at 5-10°C for 6 hours, and filtered after the reaction was completed. The liquid is the potassium iodide solution generated by the reaction, which is directly used for the iodine displacement reaction. After the solid was dried, 4.07 kg of p-iodoaniline was obtained, and the nuclear magnetic resonance test showed that it was the target product with a yield of 93.0%.
[0042] Add 4.07kg of p-iodoaniline (18.6mol) into 80 liters of water, control the reaction temperature to be less than 0°C, add 20 liters of 36.5% (w / w) concentrated hydrochloric acid with a molar ratio of hydrochloric acid to p-iodoaniline of 12.8:1, and quickly cool Precipitate ultra-fine white crystals at -10°C, then add dropwise 6.6 liters of 20% (w / w) sodium nitrite solution with a molar ratio of so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com