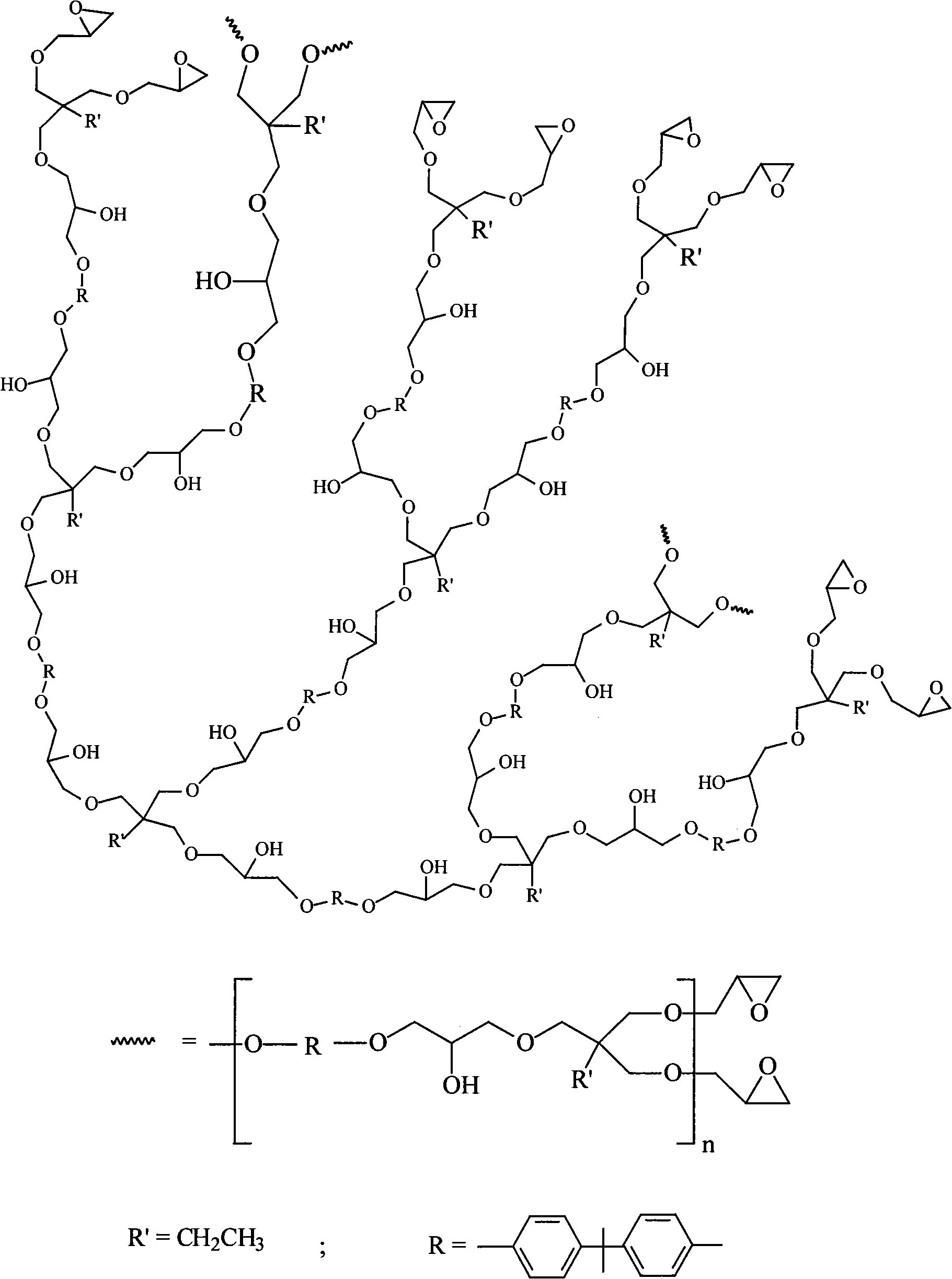
Polyether type hyperbranched epoxy resin and preparation method thereof
An epoxy resin and polyether-based technology, which is applied in the field of polyether-type hyperbranched epoxy resin and its preparation, can solve the problems of popularization and application limitations, difficult product purification, cumbersome preparation and purification process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: 4.56g (0.02mol) of bisphenol A, 6.62g (0.02mol) of trimethylolpropane triglycidyl ether, 0.6g of tetrabutylammonium chloride, and 40ml of N,N-dimethylformamide were added In the reaction kettle, react at 60°C for 15 hours under the protection of nitrogen, precipitate in ethanol after cooling, and dry to obtain a nearly colorless hyperbranched epoxy resin. The weight-average molecular weight of the product is 8452 and the molecular weight polydispersity index is 1.66 as measured by GPC.
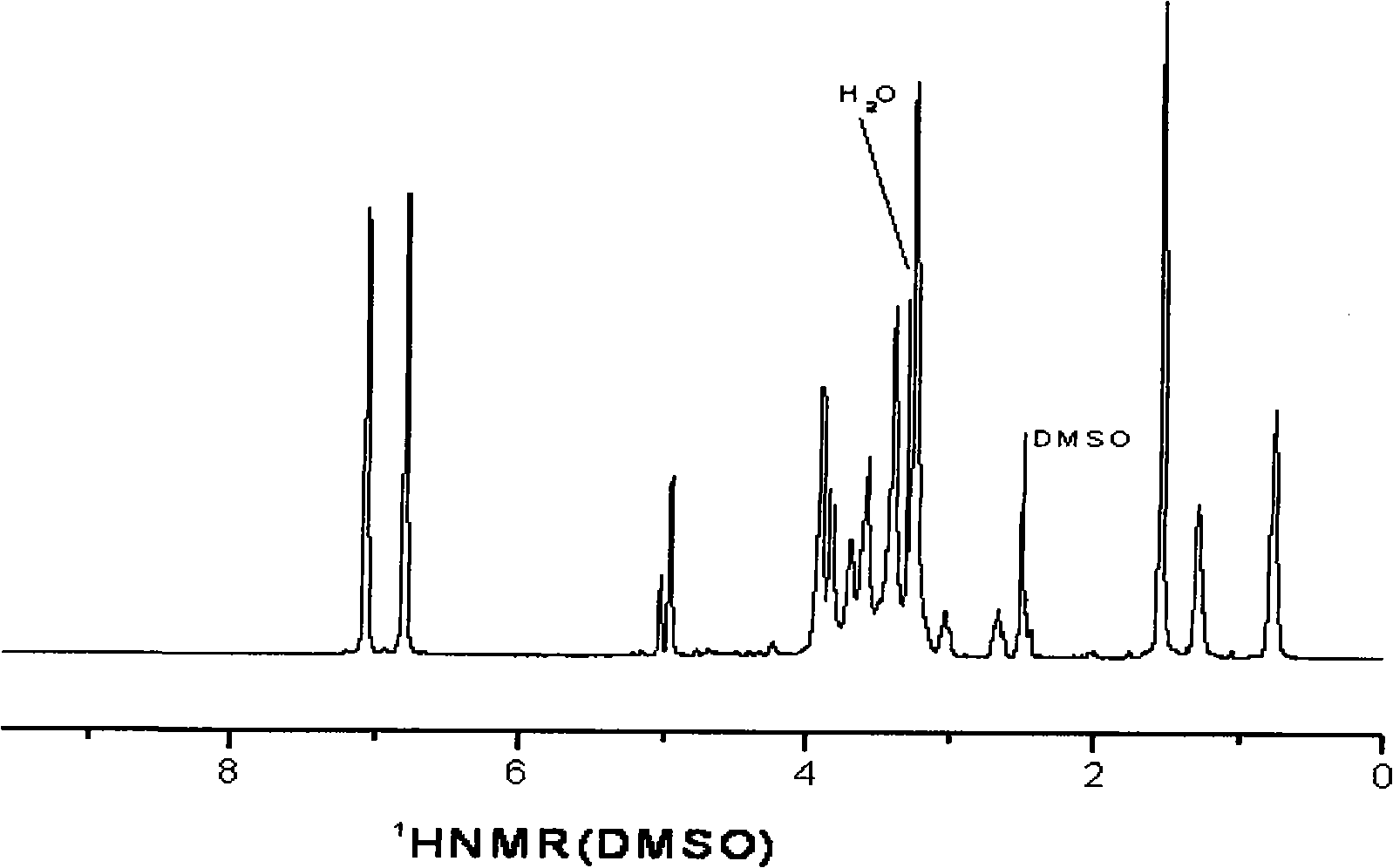
[0020] figure 2 It is the nuclear magnetic hydrogen spectrogram of the hyperbranched epoxy resin that embodiment 1 obtains 1 HNMR (DMSO), δ: 0.765 (-CH 2 CH 3 ), 1.28 (-CH 2 CH 3 ), 1.541(-Φ-C(CH 3 ) 2 -Φ), 2.512 (epoxy, -CH 2 )2.625 (epoxy, -CH), 3.000 (epoxy, -OCH 2 ), 3.262-3.899 (-OCH 2 , -CH(OH)), 4.960, 5.029 (-OH), 6.631-7.068 (aromatic-H). From this spectrum, the polymer has both the structures of bisphenol A and trimethylolpropane triglycidyl ether. In ad...
Embodiment 2
[0021] Embodiment 2: Bisphenol F 6g (0.03mol), pentaerythritol triglycidyl ether 6.36g (0.02mol), tetrabutylammonium hydroxide 1.5g, N, N-dimethyl sulfoxide 40ml add in the reactor, nitrogen Under protection, react at 120°C for 96 hours, after cooling, precipitate in a mixture of methanol and water (volume ratio 1:1), and dry to obtain a nearly colorless hyperbranched epoxy resin. The weight-average molecular weight of the product measured by GPC is 87452, which is more The dispersion index was 2.15.
Embodiment 3
[0022] Example 3: Add 1 g (0.01 mol) of hydroquinone, 14.4 g (0.05 mol) of trimethylolethane triglycidyl ether, 0.725 g of potassium tert-butoxide, and 40 ml of tetrahydrofuran into the reactor, and react for 40 After reacting at ℃ for 80 hours, after cooling, it was precipitated in methanol and dried to obtain a nearly colorless hyperbranched epoxy resin. The weight-average molecular weight of the product was 50915 as measured by GPC, and the molecular weight polydispersity index was 1.96.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com