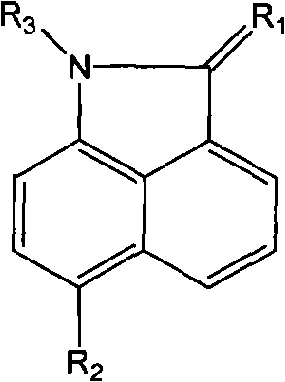
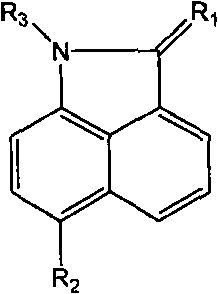
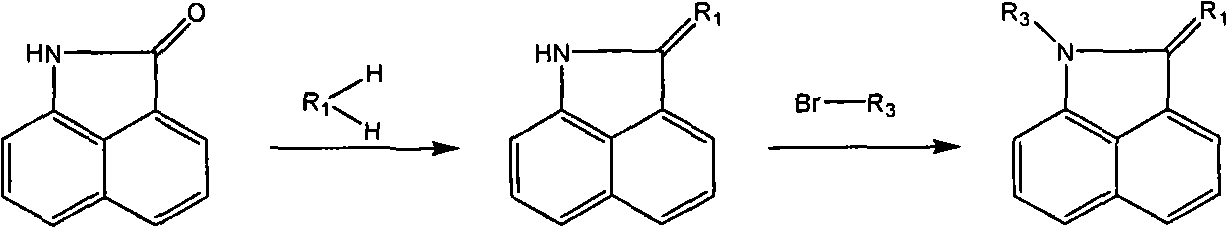
Naphthaline lactam derivative and application thereof in propagation suppression of tumor cells
A technology of tumor cell proliferation and naphthalene lactam, applied in the field of medicinal chemistry, can solve the problems that the inherent structure of naphthalene lactam is not easy to modify, the number of naphthalene lactam derivatives is limited, etc., and achieve good activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of 2-[(1-propargyl-benzo[c,d]indole)-2(1H)-ylidene]malononitrile (derivative 1):
[0035] (1) 1.69g naphthalene lactam (0.01mol), 0.6g malononitrile (0.01mol), 1.1mLPOCl 3 Add it to a 25mL two-necked bottle, add 15mL of toluene, heat to 100°C under magnetic stirring, and react at constant temperature for 4 hours, pour the reaction solution into methanol, and directly suction filter after cooling to obtain a brown solid crude product. After vacuum drying, the crude product was separated by column chromatography to obtain 1.82 g of yellow spongy crystals with a yield of 83%. Melting point: >300°C.
[0036] ESI-HRMS (m / z): Calculated: 217.064, Found: 217.0639.
[0037] 1 HNMR (d6-DMSO, 400MHz): δ (ppm): 5.30 (s, 1H), 7.21 (d, J = 7.2Hz, 1H), 7.58 (t, J 1 =7.6Hz,J 2 =8.0Hz, 1H), 7.68(d, J=8.0Hz, 1H), 7.83(t, J 1 =8.0Hz,J 2 =7.2Hz, 1H), 8.17(d, J=8.0Hz, 1H), 8.55(d, J=8.0Hz, 1H).
[0038]
[0039] (2) Dissolve 0.217g derivative 13 (1mmol) in 10mL DMF, add...
Embodiment 2
[0044] Synthesis of 2-[(1-bromoethyl-benzo[c,d]indole)-2(1H)-ylidene]malononitrile (derivative 2):
[0045]
[0046] Dissolve 0.217g derivative 13 (1mmol) in 10mL anhydrous acetonitrile, add 0.2g K 2 CO 3 , 1mL of dibromoethane, heated to reflux under magnetic stirring, the reaction was complete after 5h, directly evaporated in vacuum to obtain a solid crude product, washed with water and dried in vacuum to obtain a yellow solid. Purified by column chromatography to obtain 0.175 g of yellow crystals with a yield of 80% and a melting point of 232.3°C.
[0047] ESI-HRMS (m / z): Calculated: 323.0058, Found: 323.0067.
[0048] 1 HNMR (CDCl3, 400MHz): δ (ppm): 3.83 (t, J 1 =6.4Hz,J 2 =6.8Hz, 2H), 4.85(t, J 1 =6.4Hz,J 2 =6.8Hz, 2H), 7.25(d, J=7.6Hz, 1H), 7.61(t, J 1 =8.0Hz,J 2 =7.6Hz, 1H), 7.70(d, J=8.0Hz, 1H), 7.82(t, J 1 =8.0Hz,J 2 =7.6Hz, 1H), 8.15(d, J=8.4Hz, 2H), 8.71(d, J=7.6Hz, 1H).
Embodiment 3
[0050] Synthesis of 2-{[(2-aminoethyl)-benzo[c,d]indole]-2(1H)-ylidene}malononitrile (derivative 3):
[0051]
[0052] Dissolve 0.217g derivative 13 (1mmol) in 10mL DMF, add 0.134gNaOCH 3 (2.4mmol), the temperature was raised to 100°C, and after half an hour of reaction, 0.243g of bromoethylamine hydrobromide (1.2mmol) was added, and the reaction was refluxed for 4h. The cooled reaction solution was poured into 150 mL of ice water, the aqueous phase was extracted with dichloromethane, and the anhydrous MgSO 4 After drying the organic phase, the crude product was obtained by rotary evaporation. Purified by column chromatography to obtain 0.176 g of yellow crystals with a yield of 68% and a melting point of 213.2°C.
[0053] 1 HNMR (d6-DMSO, 400MHz): δ (ppm): 1.84 (t, NH 2 , 2H), 2.54(m, NCH 2 CH 2 N, 2H), 2.87 (m, NCH 2 CH 2 N, 2H), 6.32(d, J=7.6Hz, 1H), 6.54(t, J 1 =7.6Hz,J 2 =8.8Hz, 1H), 7.54(d, J=8.8Hz, 1H), 7.34(d, J=7.6Hz, 1H), 7.35(t, J 1 =7.6Hz,J 2 =7.2Hz,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com