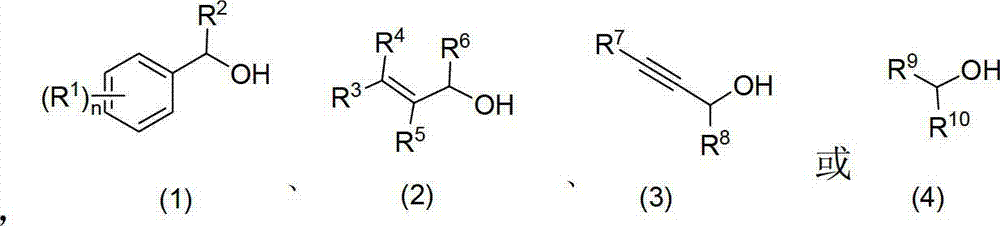
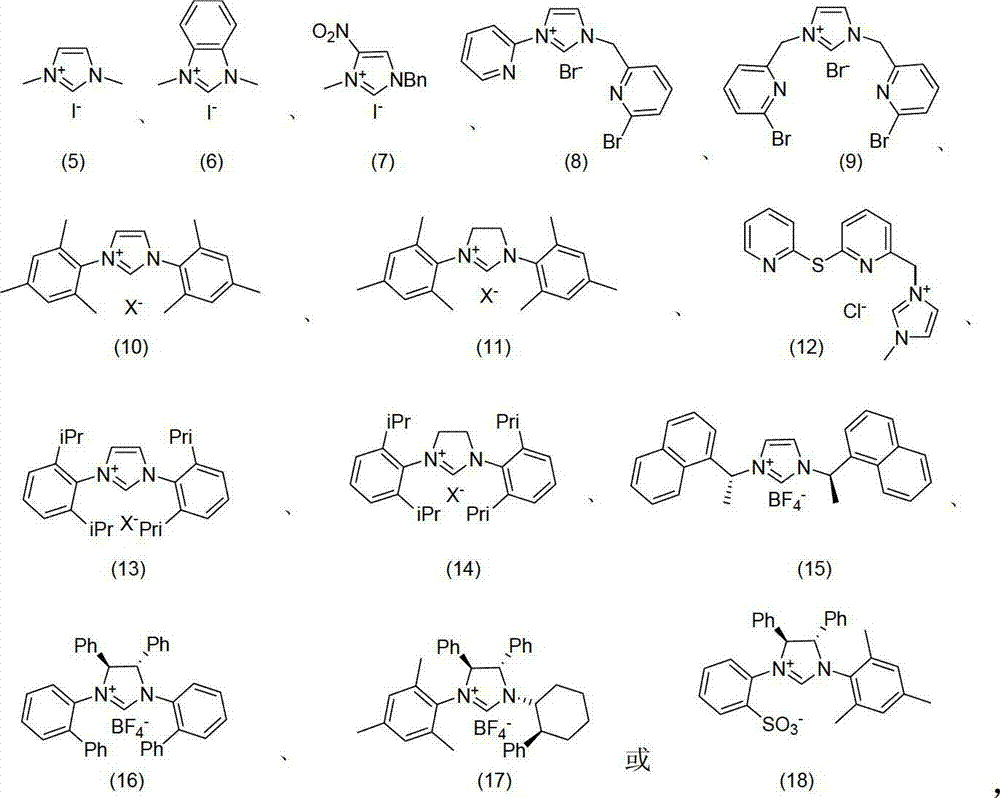
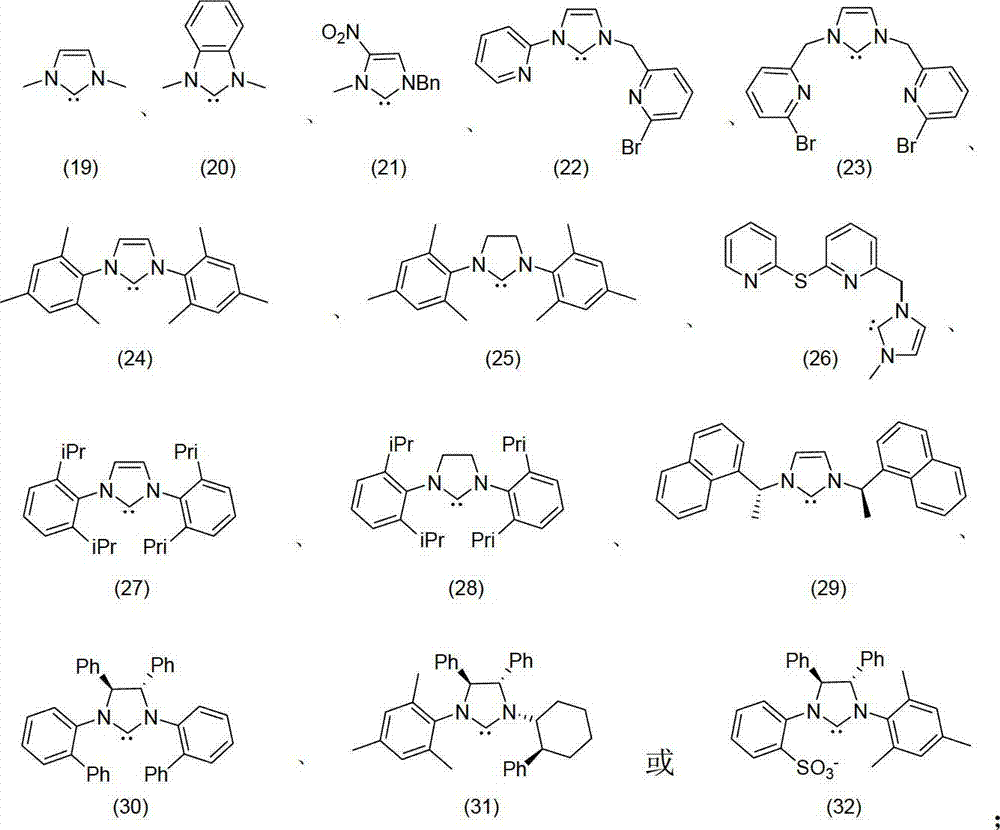
Method for oxidizing alcohol into aldehyde, ketone or carboxylic acid
A carboxylic acid and oxidizing technology, which is applied in the direction of organic chemical methods, chemical instruments and methods, and carboxylate preparation, can solve the problems of severe reaction process and troubles, and achieve good repeatability, easy operation, and easy reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add p-methoxybenzyl alcohol (1mmol), potassium tert-butoxide (0.1mmol) and imidazolium salt (9) (10μmol) into the reaction tube, vacuumize the system and flush it with air, add 10mL of toluene as a solvent, add a few capsules Molecular sieves and tetra-n-butylammonium iodide (0.05 mmol) were stirred at room temperature for 12 hours to obtain the product p-methoxybenzaldehyde with a yield of 95%.
[0037] 1 H NMR (300MHz, CDCl 3 )δ9.82(s,1H),7.78(d,J=8.7Hz,1H),6.94(d,J=8.6Hz,2H),3.82(s,3H);ESI-MS[M+Na]m / z159.0.
Embodiment 2
[0039] Add p-4-hydroxy-3-methoxybenzyl alcohol (1mmol), triethylamine (1.5mmol), metal nitrogen heterocyclic carbene complex [Ag(25) 2 ] + BF 4 - (20μmol), pure oxygen after the system was evacuated, added 8mL of anhydrous dichloromethane as a solvent, added 25mg of anhydrous sodium sulfate and tetra-n-butylphosphonium chloride (15μmol) and stirred at 45°C for 24 hours to obtain 4-hydroxy-3 -Methoxybenzaldehyde (i.e. vanillin), 92% yield.
[0040] 1 H NMR (300MHz, CDCl 3 )δ9.77(s,1H),7.56–7.30(m,2H),7.00(d,J=8.5Hz,1H),6.81(s,1H),3.89(s,3H); ESI-MS[M ]m / z152.0.
Embodiment 3
[0042] Add geraniol (1mmol), potassium hydroxide (2mmol), iron powder, imidazolium salt (12) (0.02mmol) and 30mg calcium chloride into the reaction tube, add 13mL DME as solvent and tetraethylammonium chloride (50μmol) , stirred at room temperature for 6 hours to obtain the product geranial (ie citral), with a yield of 99%.
[0043] 1 H NMR (300MHz, CDCl 3 )δ9.91(dd,J=30.1,8.2Hz,1H),5.84(d,J=8.0Hz,1H),5.05(d,J=7.9Hz,1H),2.56(t,J=7.4Hz, 1H),2.37–2.08(m,4H),1.95(s,2H),1.65(s,3H),1.57(d,J=4.9Hz,3H); EI-MS[M+H]m / z153. 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com