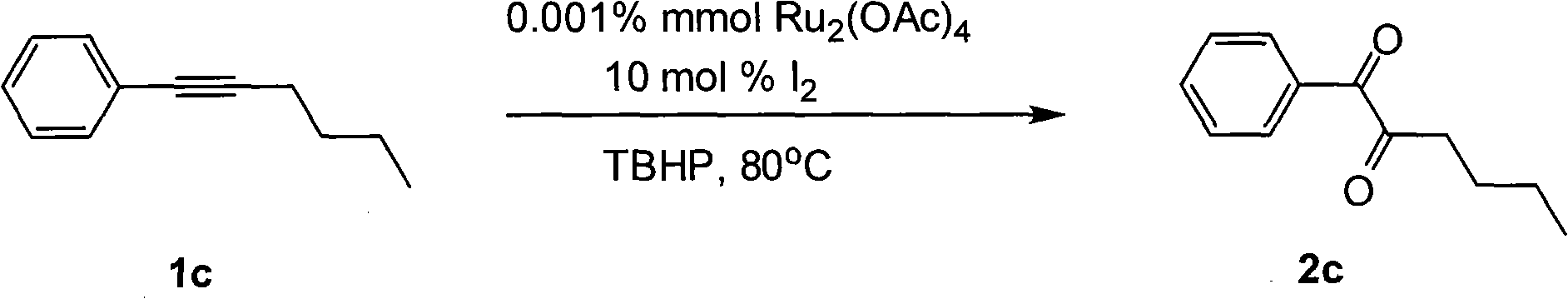Method for preparing 1, 2-diketone by catalyzing and oxidizing alkynes
A technology for catalytic oxidation and alkynes, which is used in the preparation of carbonyl compounds by oxidation, chemical instruments and methods, preparation of organic compounds, etc., to achieve the effects of wide range of use, excellent yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
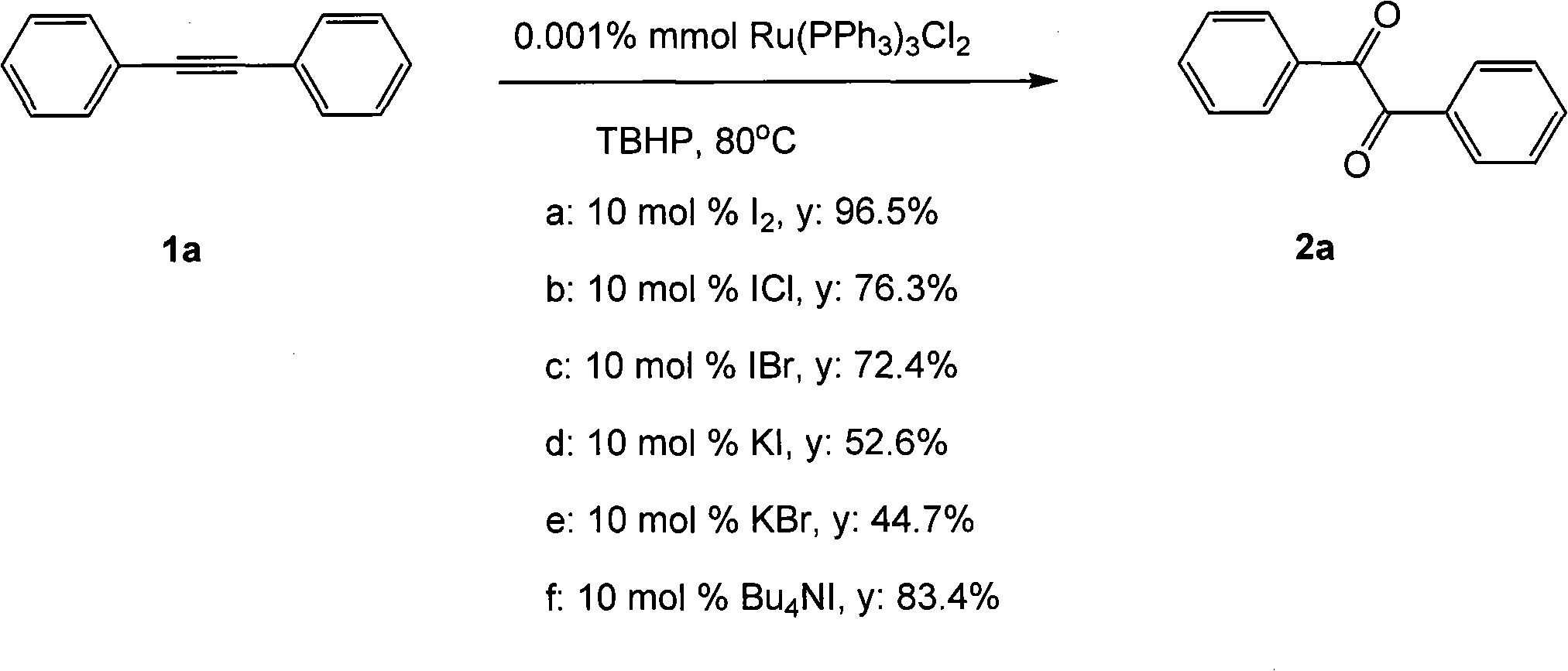
[0022]
[0023] Ru(PPh 3 ) 3 Cl 2 (0.001% mmol), tert-butanol peroxide (7.3 mmol), compound 1a (2 mmol, 356 mg), 1,4-dioxane (10 mL). Then the system was heated in an oil bath at 80°C in the air for about 12 hours, extracted with ethyl acetate (40mL×3), and the oxidation product 2a could be obtained by simple column chromatography. When the cocatalyst was selected as 10mol% I 2 (condition a), the yield is 96.5%; when using 10mol% ICl (condition b), the yield is 76.3%; when using 10mol% IBr (condition c), the yield is 72.4%; when using 10mol% KI (condition d), the yield is 52.6%; when using 10mol% KBr (condition e), the yield is 44.7%; when using 10mol%Bu 4 NI (condition f), yield 83.4%.
[0024] mp: 94-95°C; 1 H NMR (CDCl 3 , 400MHz): δ7.48-7.51(m, 4H), 7.62-7.66(m, 2H), 7.96-7.98(m, 4H); 13 C NMR (CDCl 3 , 100MHz): δ128.9, 129.8, 132.8, 134.9, 194.5; MS (C 14 h 10 o 2 ): 210; IR (KBr, cm -1 ): v1660.
Embodiment 2
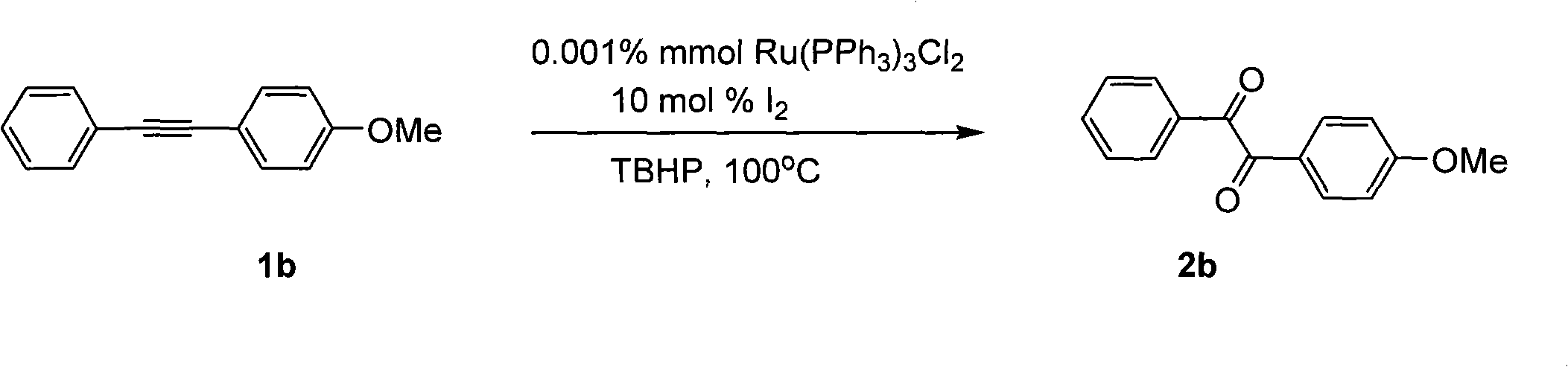
[0026]
[0027] Ru(PPh 3 ) 3 Cl 2 (0.001% mmol), iodine (0.2 mmol, 51.0 mg), compound 1b (2 mmol, 417 mg), tert-butanol peroxide (7.3 mmol), 1,4-dioxane (10 mL). Then the system was heated in an oil bath at 100° C. in air for about 10 hours, extracted with ethyl acetate (40 mL×3), and the oxidation product 2b was obtained by simple column chromatography with a yield of 93.5%. Yellow solid; mp: 53-54°C; Yield: 96%; 1H NMR (CDCl 3 , 400MHz): δ3.86(s, 3H), 6.95-6.99(m, 2H), 7.46-7.51(m, 2H), 7.61-7.63(m, 1H), 7.92-7.98(m, 4H); 13C NMR (CDCl 3 , 100MHz): δ55.5, 114.3, 125.8, 128.9, 129.7, 132.2, 133.0, 134.7, 164.9, 193.1, 194.8; MS(C 15 h 12 o 3 ): 240. IR (KBr, cm-1): v 1677.
Embodiment 3
[0029]
[0030] Ru 2 (OAc) 4 (0.001% mmol), iodine (0.2 mmol, 51 mg), compound 1c (2 mmol, 316 mg), tert-butanol peroxide (7.3 mmol), 1,4-dioxane (10 mL). Then the system was heated in an oil bath at 80°C in air for about 12 hours, extracted with ethyl acetate (40 mL×3), and the oxidation product 2c was obtained by simple column chromatography with a yield of 68.8%. 1 HNMR (CDCl 3 , 300MHz): δ0.94(t, 3H, J=7.2Hz), 1.37-1.45(m, 2H), 1.64-1.71(m, 2H), 2.86-2.91(m, 2H), 7.50(t, 2H , J=7.2Hz), 7.63(d, 1H, J=9Hz), 7.98(d, 2H, J=7.2Hz); 13 C NMR (CDCl 3 , 300MHz): δ13.79, 22.30, 24.88, 38.49, 128.84, 130.11, 131.96, 134.56, 182.60, 203.55; MS(C 12 h 14 o 2 ): 190.0994; IR (KBr, cm -1 ): v 1712.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com