Electrochemical deblocking solution for electrochemical oligomer synthesis on an electrode array
a technology of electrochemical oligomer and electrode array, which is applied in the direction of bulk chemical production, liquid/fluent solid measurement, peptides, etc., can solve the problems of deblocking solution, inability to aqueous deblock solution, and less desirable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0128] One preferred deblocking solution comprises 1 M hyrdroquinone, 10 mM benzoquinone, 50 mM tetraethyl ammonium p-toluene sulfonate, 5 mM 2,6-lutidine, 20% methanol, 80% acetonitrile. The solution is made by first mixing the methanol and acetonitrile. After adding all components, approximately one liter of solution was obtained because there was a significant increase in volume owing to the amount of hydroquinone in solution. Benzoquinone was added first, then hydroquinone, then the salt, and then lutidine. The benzoquinone was added first because it will not dissolve adequately if not added first. The solution was mixed using a stir bar on a stir plate. Mixing was performed until all components were dissolved. Mixing continued until the solution was required to be used.
example 2
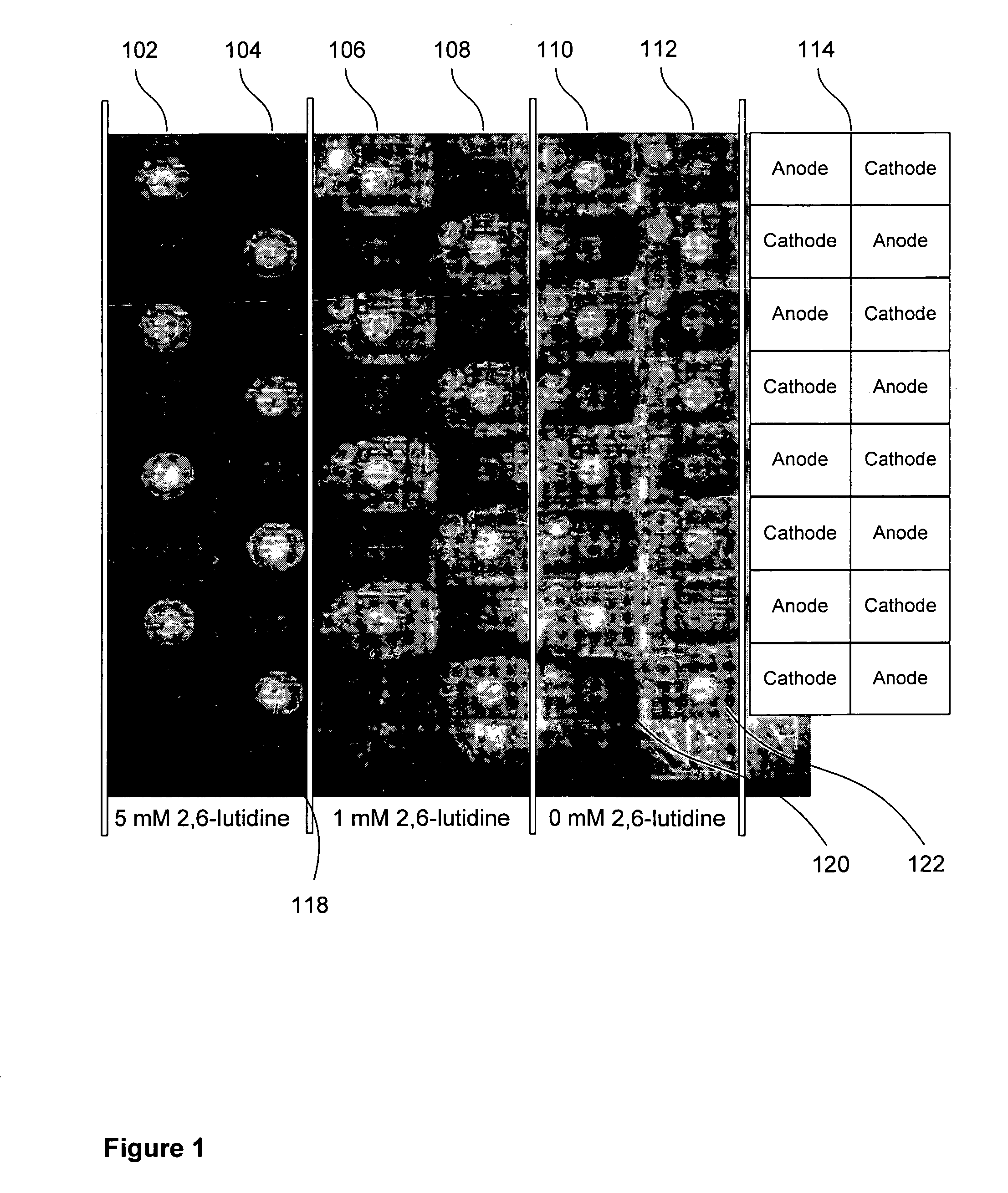
[0129] Electrochemical deblocking solutions were made in accordance with the present invention to test the effectiveness of organic base for confinement of deblocking to the anodes. The procedure comprised using the coupling of Cy3 (fluorescent) phosphoramidite to detect the efficiency and quality of dimethoxytrityl (DMT) removal by acid generated electrochemically in the presence of an electrochemical deblocking solution containing selected amounts of lutidine. Specifically, electrode microarray chips were initialized with a 5-mer oligonucleotide containing a DMT blocked 5′ hydroxyl group. Acid was generated electrochemically over specific electrodes to remove the DMT blocking group in the presence of varying amounts of lutidine. A Cy3 phosphoramidite was coupled to any non-DMT-blocked 5′ hydroxyls allowing one to visualize electrochemical deblocking using a fluorescent microscope.
[0130] The same oligonucleotide was synthesized at each electrode (ACTGT, 5′→3′). The DMT was left on...
example 3
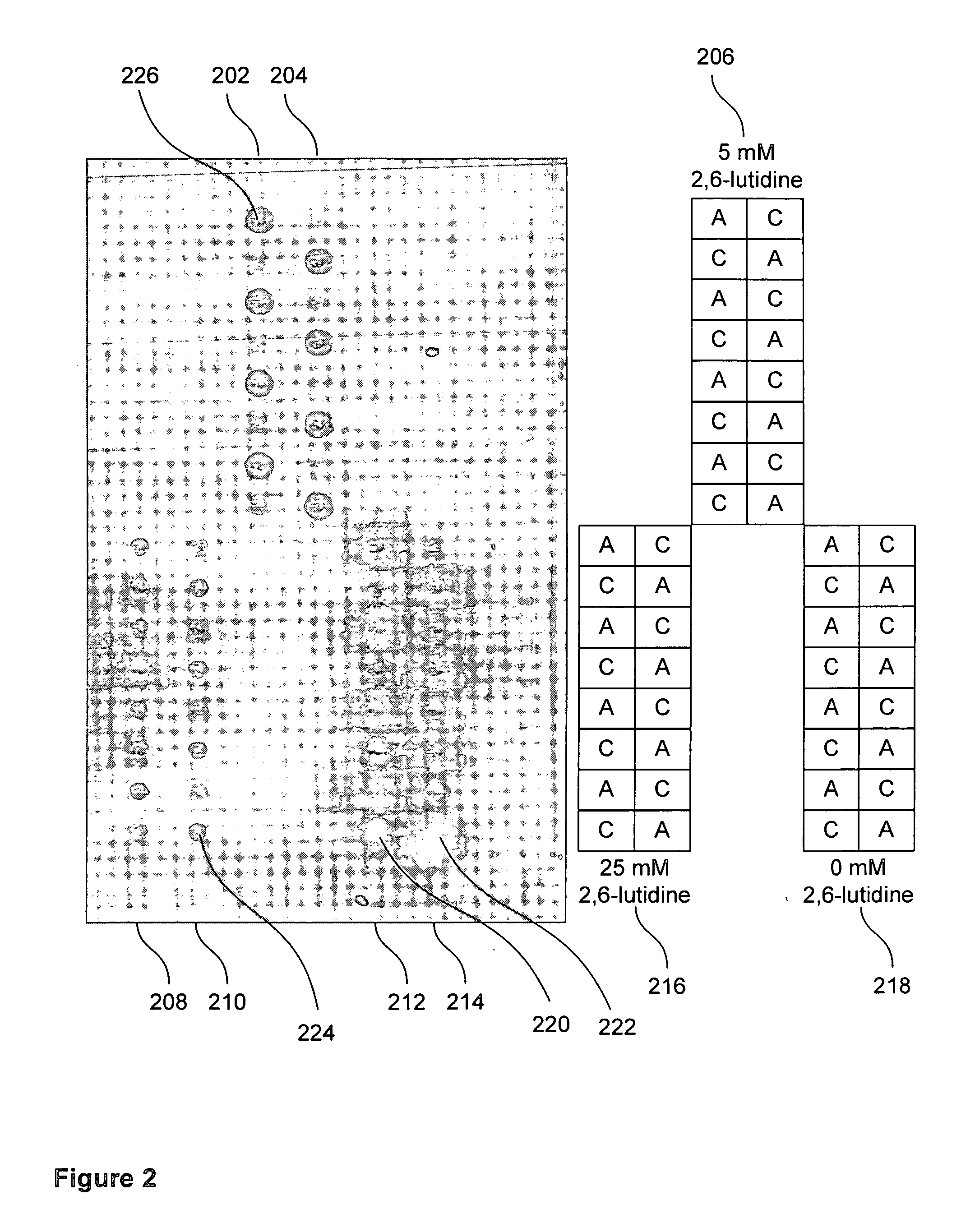
[0132] Electrochemical deblocking solutions were made in accordance with the present invention to test the effectiveness of organic base for confinement of deblocking to the anodes. The procedure comprised using the coupling of Cy3 phosphoramidite to detect the efficiency and quality of dimethoxytrityl (DMT) removal by acid generated electrochemically with electrochemical deblocking solution containing selected amounts of lutidine. Specifically, electrode microarray chips were initialized with a 5-mer oligomer containing a DMT blocked 5′ hydroxyl. Acid was generated electrochemically over specific electrodes to remove the DMT in the presence of varying amounts of lutidine. A Cy3 phosphoramidite was coupled to any non-DMT-blocked 5′ hydroxyls allowing one to visualize electrochemical deblocking using a fluorescent microscope.
[0133] The same oligonucleotide was synthesized at each electrode (ACTGT, 5′→3′). The DMT blocking group was left on the final “A” nucleotide base for testing t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| voltage | aaaaa | aaaaa |
| voltage | aaaaa | aaaaa |
| current | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com