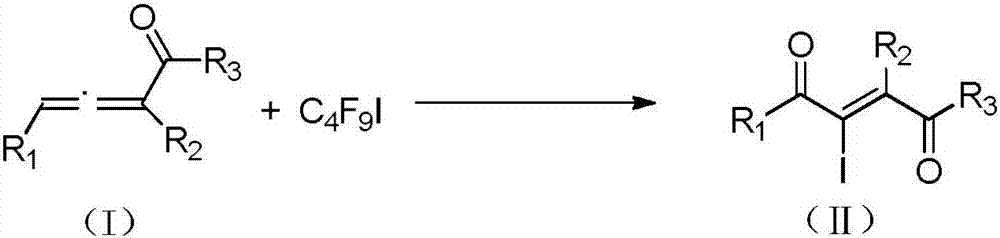Method for preparing 2-iodine amyl -2-ene-1,4-diketone derivative by adopting visible light catalysis
A catalytic preparation and visible light technology, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds, can solve problems such as poor regioselectivity, low product yield, and narrow range of reaction substrates. Gentle, good yield, high selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
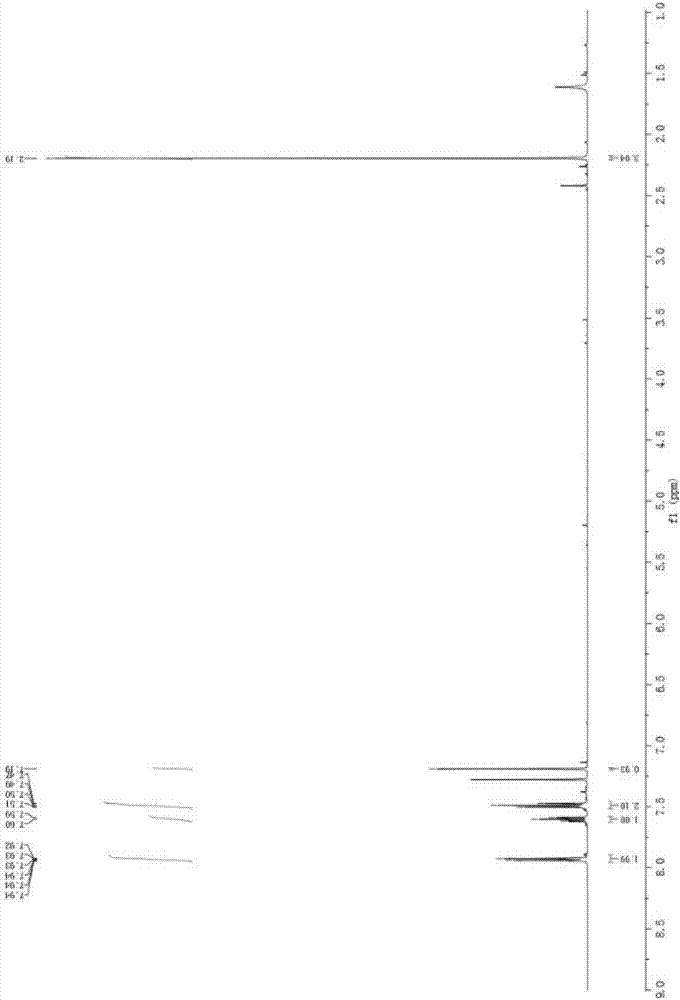
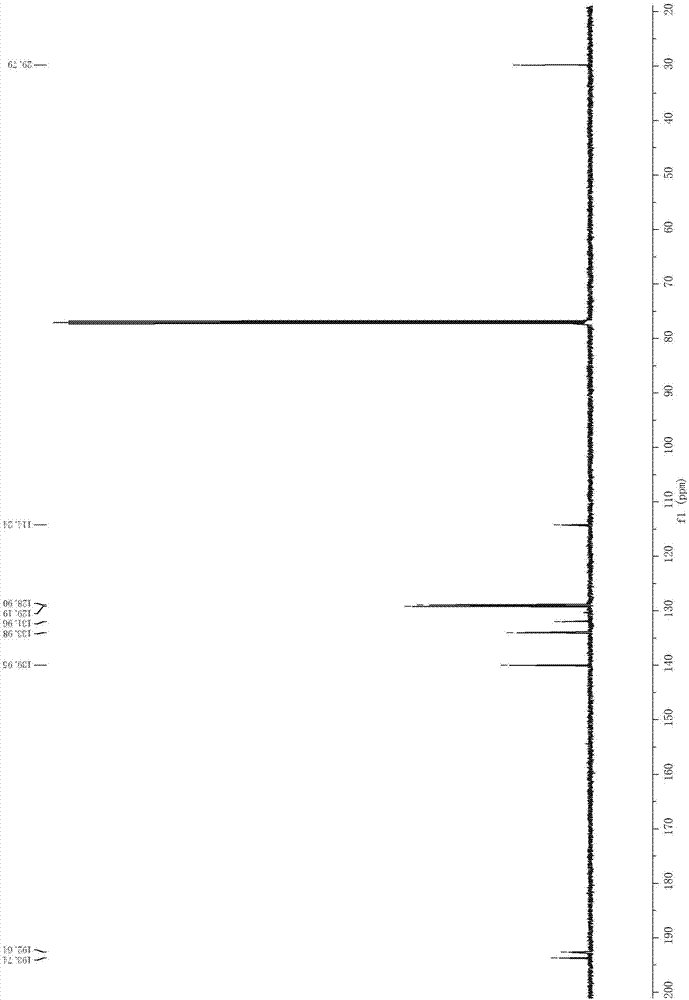
[0045] In a Schlenk tube, add NaI (60.0 mg, 0.4 mmol), Ir (ppy) in sequence 3 (1.3mg, 0.002mmol), 5-phenylpenta-3,4-dien-2-one (31.6mg, 0.2mmol), C 4 f 9 I (103.8 mg, 0.3 mmol) and toluene (6 ml) were connected to an oxygen atmosphere, and stirred at room temperature for 10 h under the irradiation of white light from a 45W energy-saving lamp. After the reaction was completed, the reaction solution was washed twice with water, and the water phase was washed with CH 2 Cl 2 Extracted twice, combined the organic phases, spin-dried by a rotary evaporator, and then purified by silica gel column chromatography to obtain the light yellow target product 2-iodo-1-phenylpent-2-ene-1,4-dione. Yield 48%, purity ≥ 98%. Product characterization data are as follows: 1H NMR (500MHz, CDCl3) δ7.93 (dt, J = 8.5, 1.5Hz, 2H), 7.63–7.58 (m, 1H), 7.52–7.46 (m, 2H), 7.19 (s, 1H), 2.19(s, 3H). 13 C NMR (126MHz, CDCl 3 )δ193.74, 192.64, 139.95, 133.98, 131.96, 129.19, 128.90, 114.24, 29.79; HRMS ...
Embodiment 2
[0048] In a Schlenk tube, sequentially add NH 4 I (58.0 mg, 0.4 mmol), Ir (ppy) 3 (1.3mg, 0.002mmol), 5-phenylpenta-3,4-dien-2-one (31.6mg, 0.2mmol), C 4 f 9 I (103.8 mg, 0.3 mmol) and toluene (6 ml) were connected to an oxygen atmosphere, and stirred at room temperature for 10 h under the irradiation of white light from a 45W energy-saving lamp. After the reaction was completed, the reaction solution was washed twice with water, and the water phase was washed with CH 2 Cl 2 Extracted twice, combined the organic phases, spin-dried by a rotary evaporator and purified by silica gel column chromatography to obtain the light yellow target product (Z)-2-iodo-1-phenylpent-2-ene-1,4 - Diketone, yield 49.6%, purity ≥ 98%.
Embodiment 3
[0050] In a Schlenk tube, add KI (66.4 mg, 0.4 mmol), Ir (ppy) in sequence 3 (1.3mg, 0.002mmol), 5-phenylpenta-3,4-dien-2-one (31.6mg, 0.2mmol), C 4 f 9 I (103.8 mg, 0.3 mmol) and toluene (6 ml) were connected to an oxygen atmosphere, and stirred at room temperature for 10 h under the irradiation of white light from a 45W energy-saving lamp. After the reaction was completed, the reaction solution was washed twice with water, and the water phase was washed with CH 2 Cl 2 Extracted twice, combined the organic phases, spin-dried by a rotary evaporator, and then purified by silica gel column chromatography to obtain the light yellow target product 2-iodo-1-phenylpent-2-ene-1,4-dione. Yield 63%, purity ≥ 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com