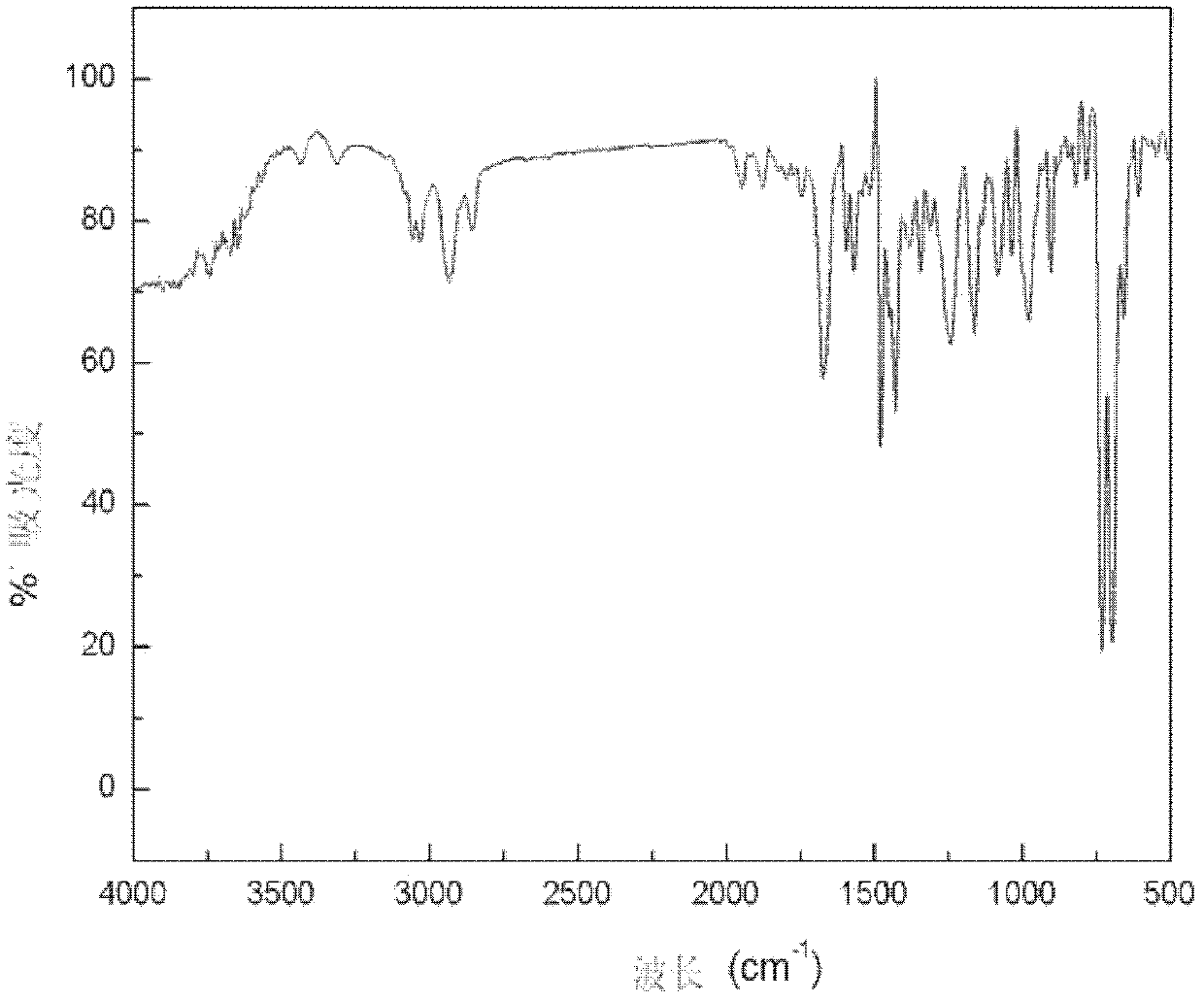
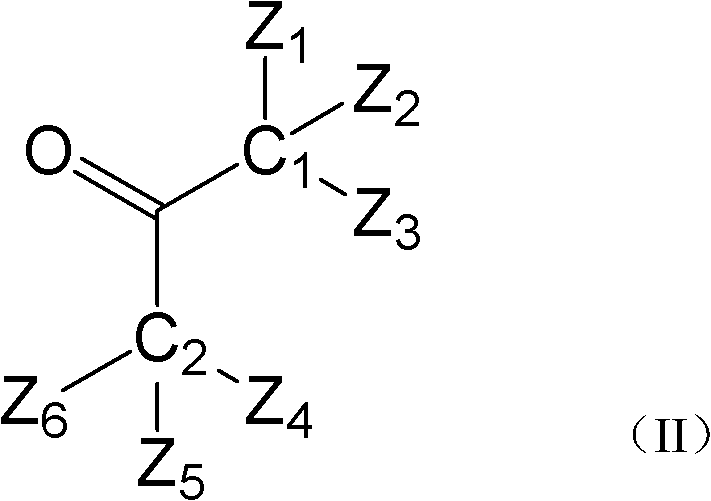
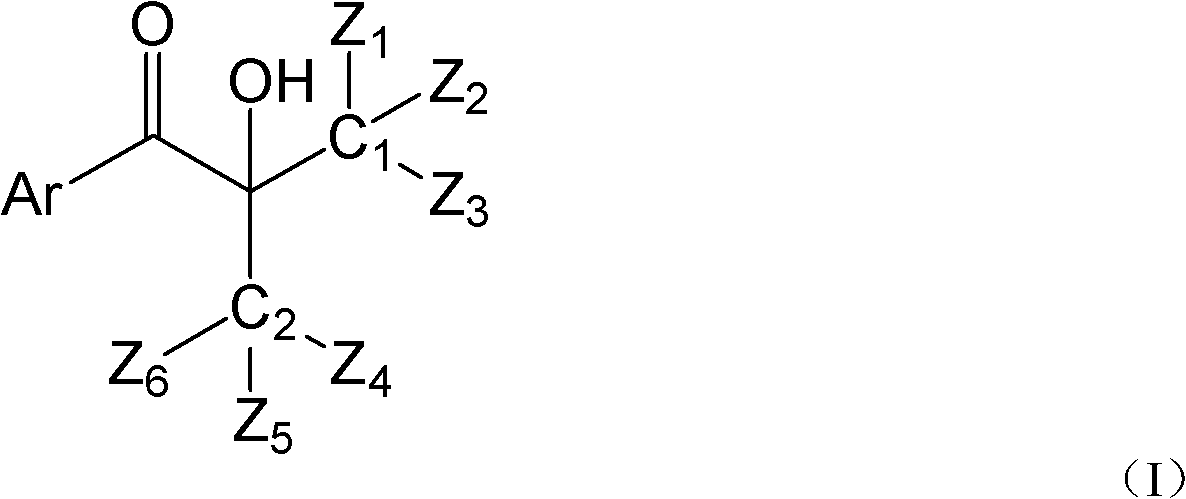Hydroxyalkyl aryl ketone photoinitiator capable of reducing volatile organic compound (VOC) discharge until elimination of VOC discharge
A technology of hydroxyalkyl aryl ketone and photoinitiator, applied in the field of ultraviolet light curing, can solve the problems of not meeting European VOC emission requirements, not meeting the requirements, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Embodiment 1: Preparation of 2-hydroxyl-2-hexyl-1-biphenyl-1-octanone
[0110]Embodiment 1 is completed by the following five-step reactions of 1A, 1B, 1C, 1D, and 1E;
[0111]
[0112] 1A. Take 32.2g of diethyl malonate and 85.2g of bromohexane in a 500ml three-necked flask equipped with a condenser, add dropwise 100ml of ethanol solution containing 33.2g of sodium ethoxide under stirring at room temperature, after the dropwise addition is complete, slowly raise the temperature To 55°C-65°C, react for 2 hours, continue to heat up and reflux for 2 hours, after the reaction is completed, desolventize at normal pressure and then reduce pressure until the temperature of the kettle reaches 130°C, after cooling down, mix the organic phase with 200ml of 10% sodium hydroxide aqueous solution, and reflux The hydrolysis was carried out for 14 hours. After the hydrolysis was completed, the reaction solution was poured into 200ml of 10% dilute hydrochloric acid, and a yellow oi...
Embodiment 2-6
[0118] The preparation methods of the compounds in Examples 2-6 are similar to those in Example 1, except that the aromatic hydrocarbons shown in Table 3 are used as starting materials in the synthesis step 1C.
[0119] Table three compounds of the present invention
[0120]
[0121]
Embodiment 7-12
[0123] The preparation methods of the compounds in Examples 7-12 are similar to those in Example 1, except that the halogenated compounds shown in Table 4 were used as starting materials in the synthesis process of 1A.
[0124] Table 4 Compounds of the present invention
[0125]
[0126]
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com