Method for synthesizing alpha,beta-unsaturated aryl ketone compound from dimethyl sulfoxide and arylethanone
A technology of dimethyl sulfoxide and aryl ethyl ketone, which is applied to the synthesis of α from dimethyl sulfoxide and aryl ethyl ketone, can solve the problems of unfavorable large-scale production, low safety and environmental protection, and complicated operation, and meet the requirements of Industrial production requirements, low cost, and high reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Aryl ethyl ketone raw material:
[0047] α,β-Unsaturated aryl ketones products:
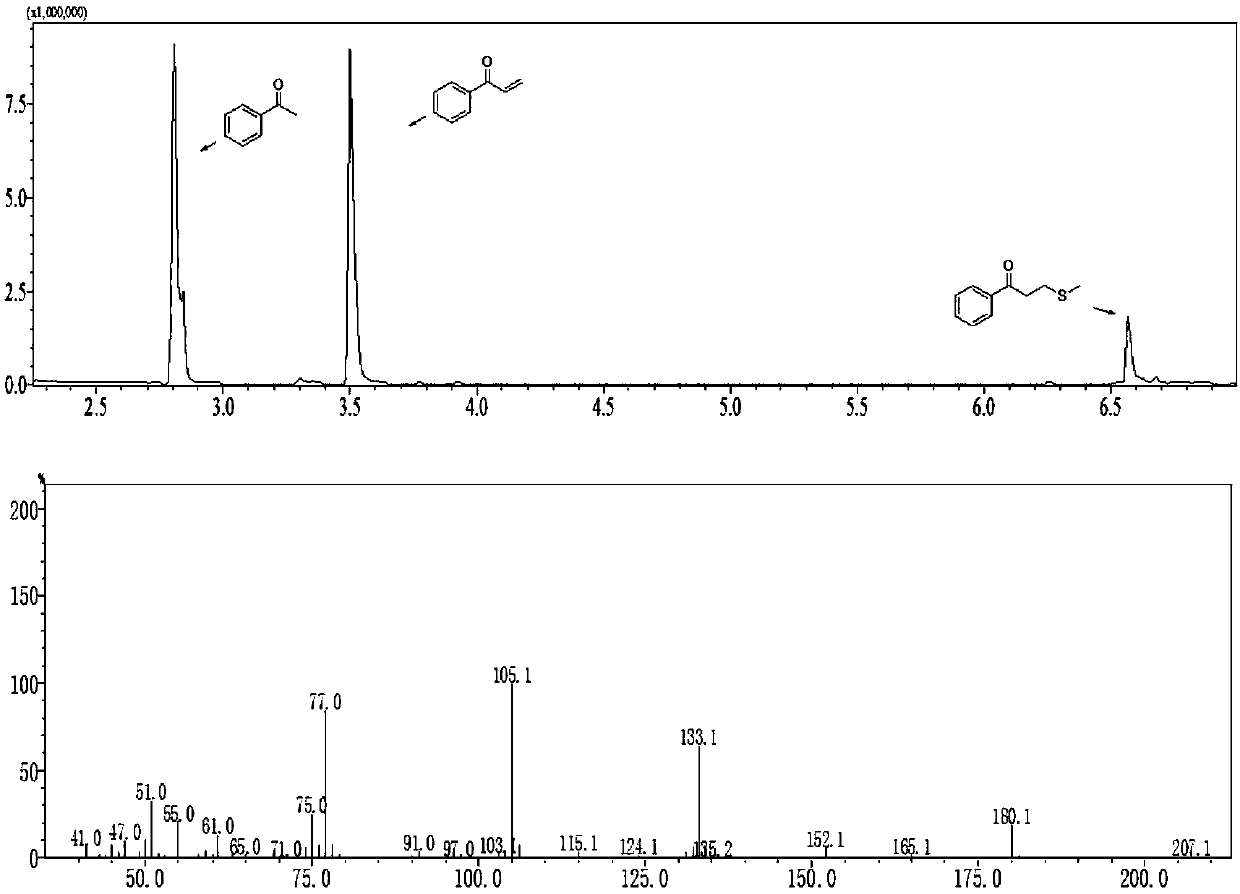
[0048] The reaction time is 9h, the product is a colorless oil, and the yield is 85%.
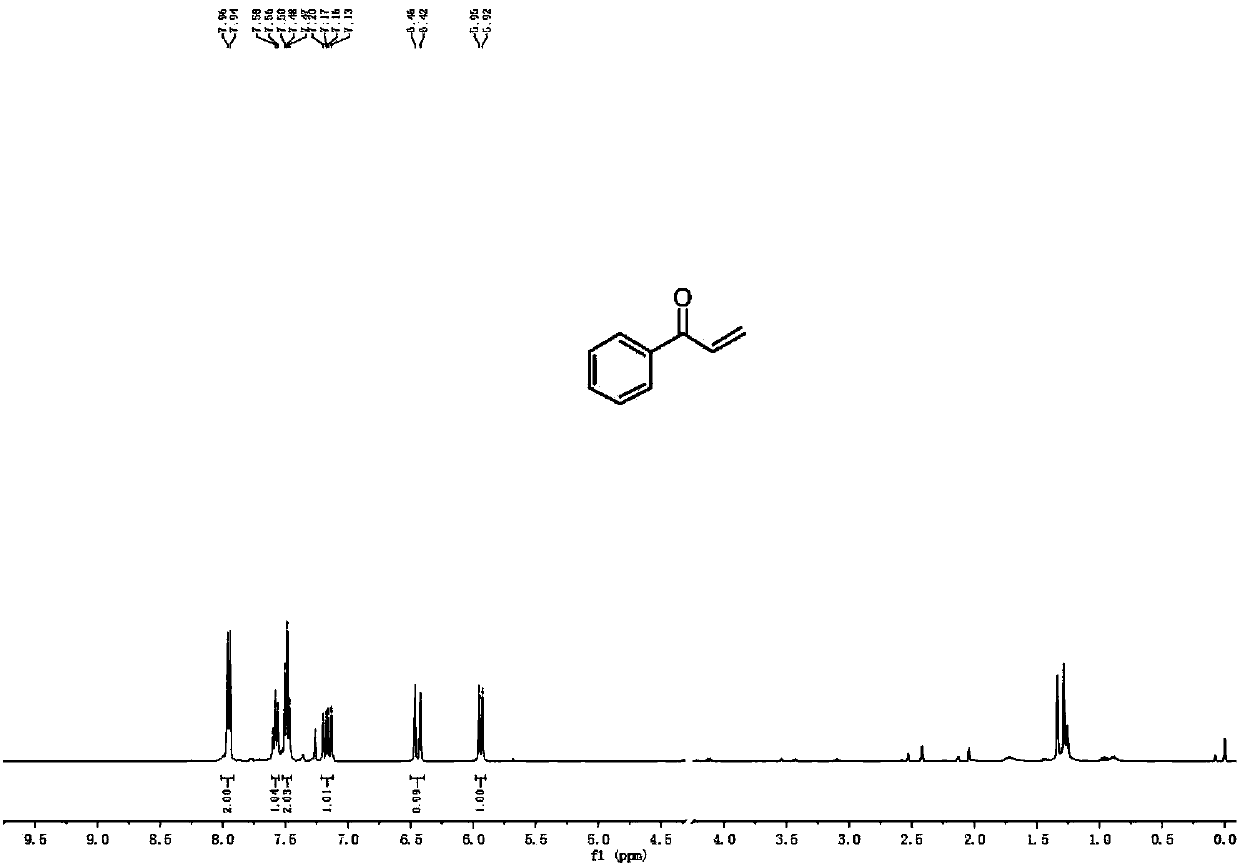
[0049] 1 H NMR (400MHz, CDCl 3 ): δ7.95(d, J=7.7Hz, 2H), 7.58(t, J=7.3Hz, 1H), 7.48(t, J=7.5Hz, 2H), 7.16(dd, J=17.1, 10.6Hz ,1H),6.44(d,J=17.1Hz,1H),5.94(d,J=10.6Hz,1H).
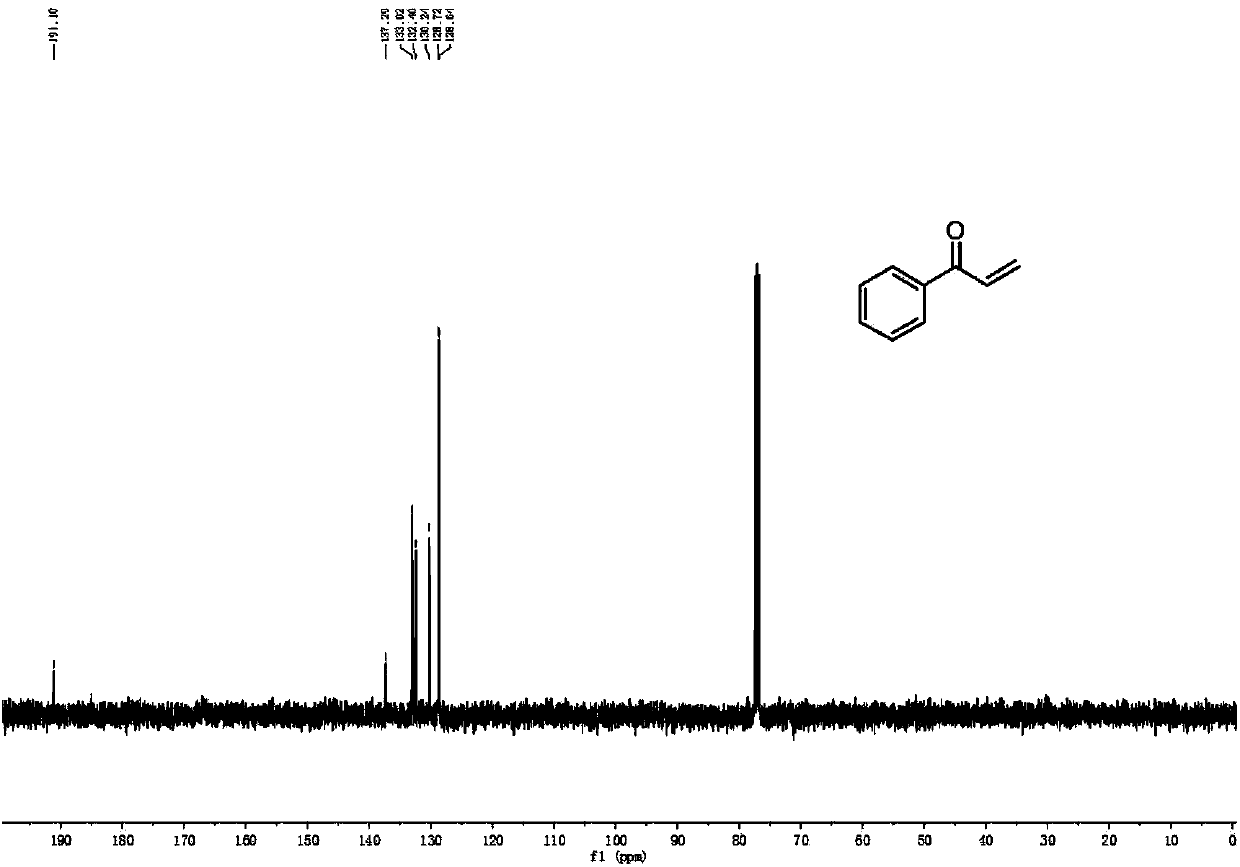
[0050] 13 C NMR (101MHz, CDCl 3 ): δ191.1, 137.2, 133.0, 132.4, 130.2, 128.7, 128.6.
Embodiment 2
[0052] Aryl ethyl ketone raw material:
[0053]α,β-Unsaturated aryl ketones products:
[0054] The reaction time is 9h, the product is light yellow oil, and the yield is 74%.
[0055] 1 H NMR (400MHz, CDCl 3 ): δ7.97(d, J=8.6Hz, 2H), 7.18(dd, J=17.0, 10.5Hz, 1H), 6.96(d, J=8.6Hz, 2H), 6.42(d, J=17.0Hz ,1H),5.87(d,J=10.5Hz,1H),3.88(s,3H).
[0056] 13 C NMR (101MHz, CDCl 3 ): δ189.2, 163.5, 132.1, 131.0, 130.2, 129.3, 113.8, 55.5.
Embodiment 3
[0058] Aryl ethyl ketone raw material:
[0059] α,β-Unsaturated aryl ketones products:
[0060] The reaction time was 9h, and the product was light yellow oil with a yield of 77%.
[0061] 1 H NMR (400MHz, CDCl 3 ): δ7.95(d, J=8.6Hz, 2H), 7.17(dd, J=17.1, 10.5Hz, 1H), 6.94(d, J=8.6Hz, 2H), 6.42(d, J=17.0Hz ,1H),5.86(d,J=10.5Hz,1H),4.10(q,J=7.0Hz,2H),1.44(t,J=7.0Hz,3H).
[0062] 13 C NMR (101MHz, CDCl 3 ): δ189.2, 163.0, 132.1, 131.0, 130.0, 129.1, 114.2, 63.7, 14.6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com