DOPO derivatives as well as preparation method and application thereof
A compound and heteroaryl technology, applied to a class of DOPO derivatives, their preparation and application fields, can solve the problems of early degradation of polymer materials, reduced thermal stability of flame retardants, and increased oxidation performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Embodiment 1 has the preparation of formula (2) structure compound
[0082] DOPO (86.40 g, 0.40 mol), acetophenone (24.05 g, 0.20 mol) and 10 ml of xylene were added to a three-necked flask equipped with a thermometer, a water separator, a magnetic stir bar and a constant pressure funnel. Under nitrogen protection, the mixture was heated to 154°C, and phosphorus oxychloride was added dropwise. POCl 3 (30.25g) was slowly added dropwise to the reaction solution within 25 hours, and the distillate was collected in the water trap, keeping the reaction temperature at 154-160°C. After dropping phosphorus oxychloride, keep warm for half an hour. After cooling, add 120g of isopropanol, stir under reflux, most of the crude product dissolves after softening, and the system becomes turbid. Stirring was stopped, cooled, and a large amount of product precipitated after standing for a period of time. Suction filtration, the solid product was first washed with a small amount of is...
Embodiment 2
[0084] Embodiment 2 has the preparation of formula (3) structure compound
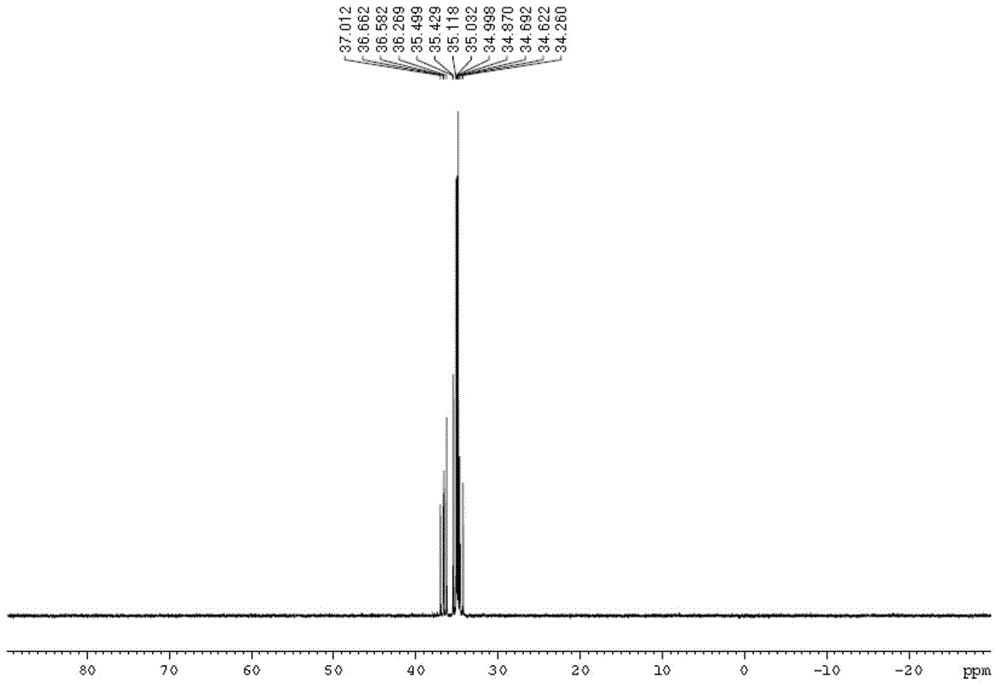
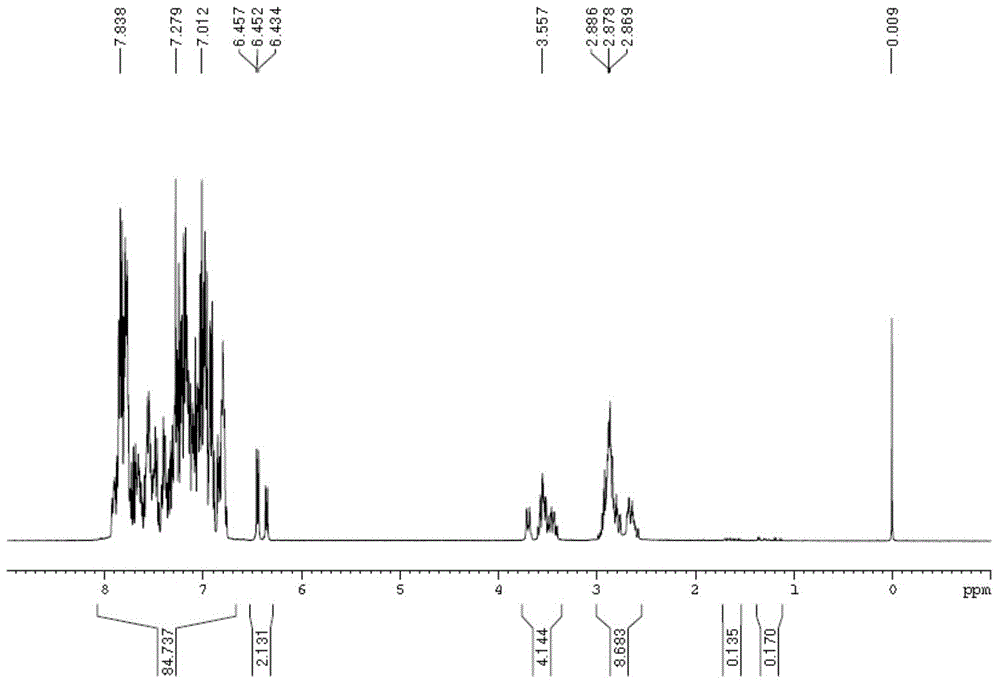
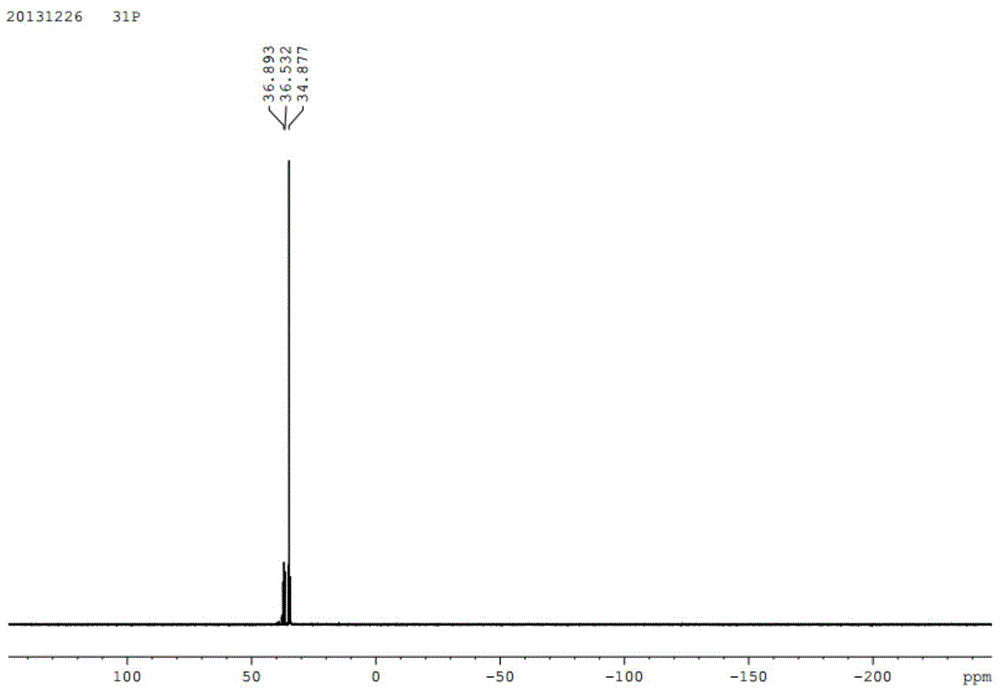
[0085] DOPO (86.40 g, 0.40 mol) and naphthalene ethyl ketone (34.04 g, 0.20 mol) were added into a three-necked flask equipped with a thermometer, a magnetic stir bar and a constant pressure funnel. Under nitrogen protection, the mixture was heated to 170°C, and phosphorus oxychloride was added dropwise. POCl 3 (15.96g) was slowly added dropwise in the reaction solution within 20 hours, keeping the reaction temperature at 170-180 degrees. After dropping phosphorus oxychloride, keep warm for half an hour. 31 P NMR spectrum showed no starting material. Cool, add 50ml of 90% ethanol to dissolve, then drop about 60ml of 2.5% Na 2 CO 3 Solution The pH of the reaction solution was adjusted to 6-7, and the white solid product was obtained by suction filtration after reflux for half an hour. Yield 70.6%.
[0086] The obtained product is characterized by H spectrum and P spectrum on a magnetic resonance ...
Embodiment 3
[0088] According to the preparation method in Example 1, phosphorus oxychloride was changed into 13.7 grams of phosphorus trichloride to obtain a compound having the structure of formula (2). Yield 75.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com