Application of palladium complex to fatty amine formylation reaction
The technology of fatty amine formyl and fatty amine formamide is applied in the application field of palladium complexes in fatty amine formylation reaction, and can solve the problems of mild reaction conditions, small adaptability of substrates, use of toxic reagents, and the like, Achieve the effect of easy separation, cheap and low toxicity separation, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
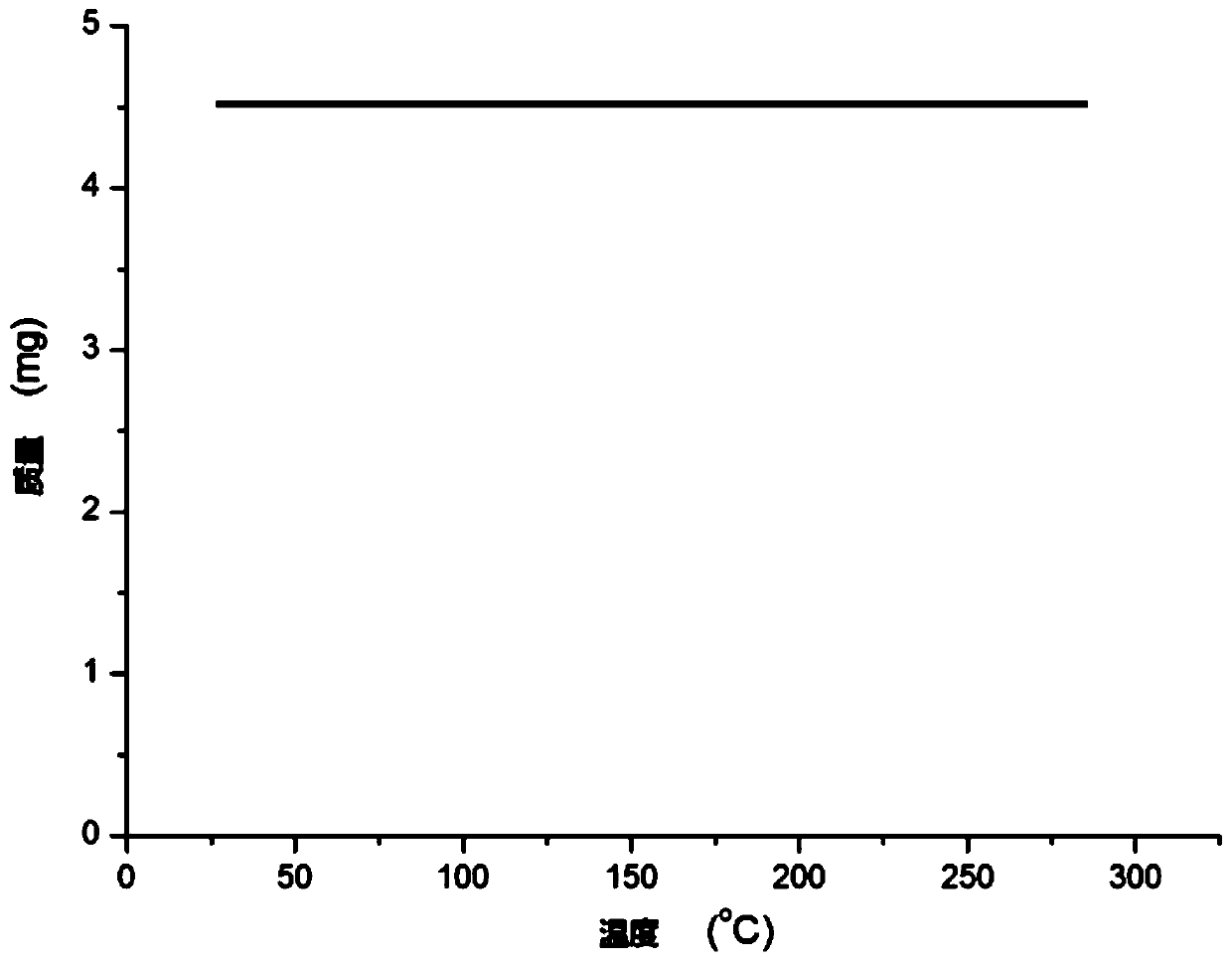
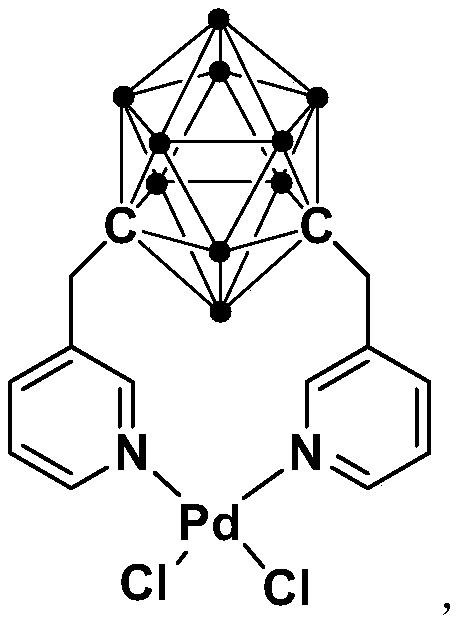
[0032] Synthesis of N,N-coordinated divalent palladium complexes Pd containing m-carborane ligands:
[0033]
[0034] At -78°C, n-BuLi (1.6M) in n-hexane (1.00 mL, 1.6 mmol) was slowly added dropwise to m-C containing ortho carborane 2 B 10 h 10 (92.0mg, 0.64mmol) in tetrahydrofuran solution, stirred at this temperature for 30 minutes, slowly rose to room temperature and continued to react for 1 hour, then added 3-chloromethylpyridine (162.3mg, 1.28mmol), continued to react at room temperature 5 hours. Then PdCl 2 (112.0 mg, 0.64 mmol) was added to the reaction system for an additional 2 hours. After the reaction is over, let it sit and filter, and dry the solvent under reduced pressure. The obtained crude product is separated by column chromatography (petroleum ether / ethyl acetate=8:1), and the brown target product palladium complex Pd (231.8 mg, produced rate of 72%).
[0035] 1 H NMR (400MHz, CDCl 3 ,25℃):δ=7.89(d,J=7.0Hz,2H),7.58(s,2H),7.42(d,J=6.5Hz,2H),7.30(t,...
Embodiment 2
[0038] Palladium complex catalyzed ethyl carbamylation reaction:
[0039]
[0040] The palladium complex Pd prepared in Example 1 was used as a catalyst to catalyze the formyl reaction of ethylamine: ethylamine (1mmol, 45mg) and divalent palladium complex Pd (0.01mmol, 5.0mg) were dissolved in 4mL of DMF, at 60 The reaction was carried out at ℃ for 10 hours. After the end, the concentrated reaction solution was directly separated by silica gel column chromatography, and dried until the mass remained unchanged to obtain the corresponding product C 3 h 7 NO (90% yield), 1 H NMR (400MHz, CDCl 3 , 25°C): δ=9.93 (s, 1H), 4.56 (s, 1H), 3.32-3.21 (m, 2H), 1.65 (t, J=7.5Hz, 3H). Elemental analysis: C 49.30, H9.65, N 19.16 (theoretical); C 49.33, H 9.68, N 19.19 (actual).
Embodiment 3
[0042] Palladium complexes catalyze the formylation of n-propylcarbamate:
[0043]
[0044]The palladium complex Pd prepared in Example 1 was used as a catalyst to catalyze the formyl reaction of n-propylamine: n-propylamine (1mmol, 59mg) and divalent palladium complex Pd (0.002mmol, 1.0mg) were dissolved in 4mL DMF, at 100 The reaction was carried out at ℃ for 8 hours, and after the end, the concentrated reaction solution was directly separated by silica gel column chromatography, dried until the mass remained constant, and the corresponding product C was obtained. 4 h 9 NO (96% yield), 1 H NMR (400MHz, CDCl 3 ,25℃):δ=9.95(s,1H),4.59(s,1H),3.36-3.25(m,2H),1.65-1.60(m,2H),1.09(t,J=7.2Hz,3H) . Elemental analysis: C 55.15, H 10.41, N 16.08 (theoretical); C 55.11, H 10.48, N 16.04 (actual).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com