A kind of method that utilizes the palladium imine complex containing pyridine ligand to catalyze the synthesis of enamine
A technology for synthesizing enamines and palladium imines, which is applied in the field of catalytic chemistry, can solve the problems of limited types of amino reagents, restricting the chemical development of enamines, single type of raw materials, etc., and achieves a variety of catalytic substrates, a simple and green synthesis process, and a catalytic effect. Efficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Divalent palladium-imine complex catalyzed the hydroamination of phenylacetylene to prepare enamines:
[0035]
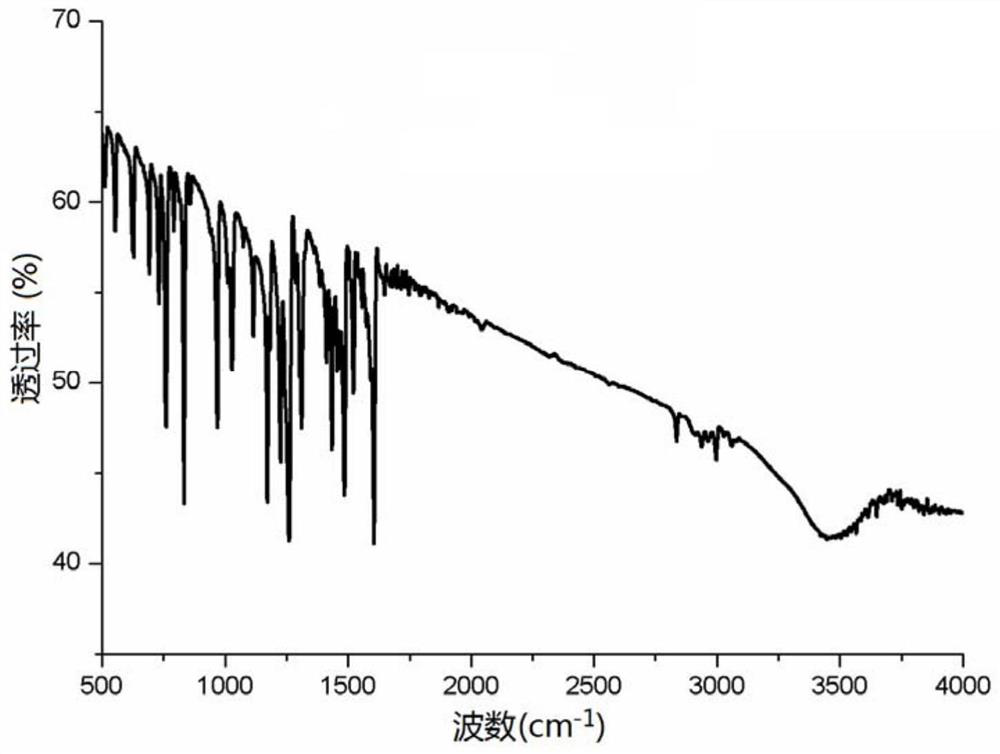
[0036] The catalyst [CH 2 (C 5 H 4 N) 2 ]Pd=NPh (3.7mg, 0.00001mol), phenylacetylene (1.02g, 0.01mol), methylphenylamine (1.02g, 0.01mol) and 6mL of toluene were added to the reaction tube, the reaction temperature was 60°C, and the reaction time was 8h , the system was extracted with ethyl acetate 3 times after the end, and the reaction solution was concentrated and separated by silica gel column chromatography to obtain the corresponding enamine compounds. The obtained product was subjected to LC-MS to obtain 1.9855 g of the product with a yield of 95%. 1 H NMR (500MHz, CDCl 3 ): δ7.88(d, J=7.0Hz, 2H), 7.63-7.51(m, 5H), 7.40-7.33(m, 3H), 6.67(s, 1H), 5.93(s, 1H), 3.05( s,3H). The catalyst used [CH 2 (C 5 H 4 N) 2 ] The infrared spectrum of Pd=NPh is shown in figure 1 .
Embodiment 2
[0038] Divalent palladium-imine complex catalyzed the hydroamination of phenylacetylene to prepare enamines:
[0039]
[0040] The catalyst [CH 2 (C 5 H 4 N) 2 ]Pd=NPh (3.7mg, 0.00001mol), phenylacetylene (1.22g, 0.012mol), methylethylamine (1.02g, 0.01mol) and 10mL of toluene were added to the reaction tube, the reaction temperature was 75°C, and the reaction time was 6h , the system was extracted with ethyl acetate 3 times after the end, and the concentrated reaction solution was separated by silica gel column chromatography to obtain the corresponding enamine compounds. The obtained product was subjected to LC-MS to obtain 1.4973 g of the product with a yield of 93%. 1H NMR (500MHz, CDCl 3 ): δ7.85(d, J=7.0Hz, 2H), 7.48-7.41(m, 3H), 6.67(s, 1H), 5.95(s, 1H), 3.11(brs, 2H), 3.05(s, 3H), 1.83 (t, J=7.0Hz, 3H). The catalyst used [CH 2 (C 5 H 4 N) 2 ]Pd=NPh infrared spectrum see figure 1 .
Embodiment 3
[0042] Divalent palladium-imine complex catalyzed the hydroamination of phenylacetylene to prepare enamines:
[0043]
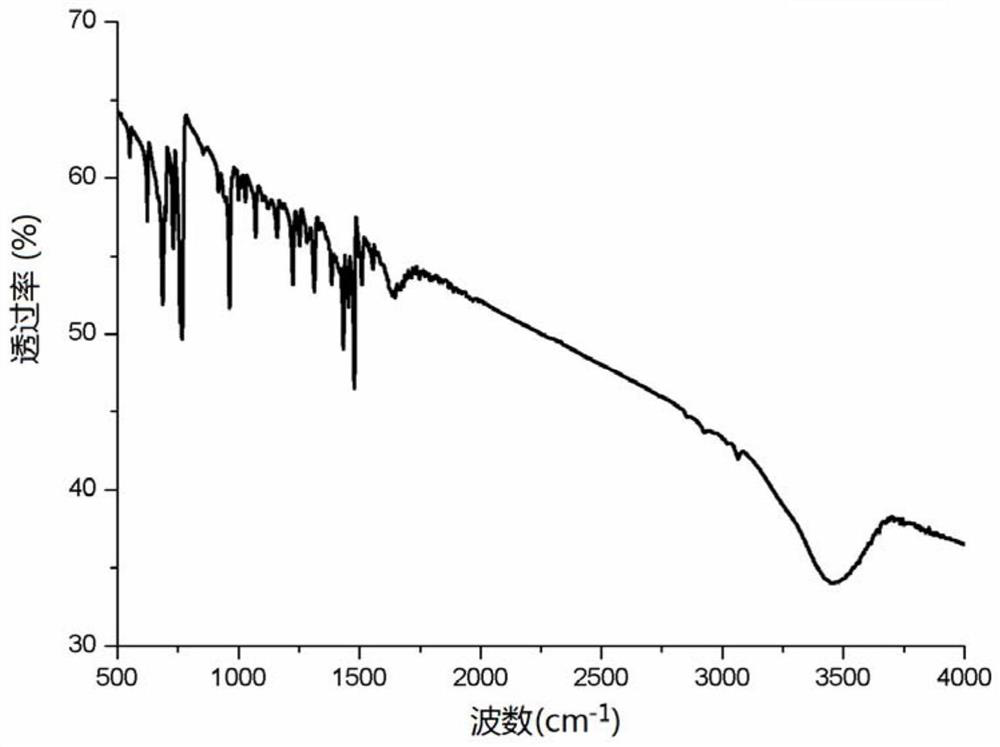
[0044] The catalyst [(CH 3 )CH(C 5 H 4 N) 2 ]Pd=NPh (3.8mg, 0.00001mol), phenylacetylene (1.53g, 0.015mol), methyl isopropylamine (1.02g, 0.01mol) and 15mL toluene were added to the reaction tube, the reaction temperature was 100°C, and the reaction time was After 12 hours, the system was extracted with ethyl acetate for 3 times, and the reaction solution was concentrated and separated by silica gel column chromatography to obtain the corresponding enamine compounds. 1H NMR (500MHz, CDCl 3 ): δ7.87(d, J=7.0Hz, 2H), 7.47-7.42(m, 3H), 6.65(s, 1H), 5.90(s, 1H), 3.15(brs, 1H), 3.06(s, 3H), 1.85(t, J=7.2Hz, 6H). The catalyst used [(CH3 )CH(C 5 H 4 N) 2 ]Pd=NPh infrared spectrum see figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com