A kind of method that catalyzes the hydroamination reaction of cyclohexylacetylene to prepare enamine compounds
An acetylene hydrogen amine and cyclohexyl technology, applied in the field of catalytic chemistry, can solve the problems of low tolerance of functional groups, limited development of enamine chemistry, limited types of amino reagents, etc., and achieves excellent high yield, simple and green synthesis process, Excellent selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
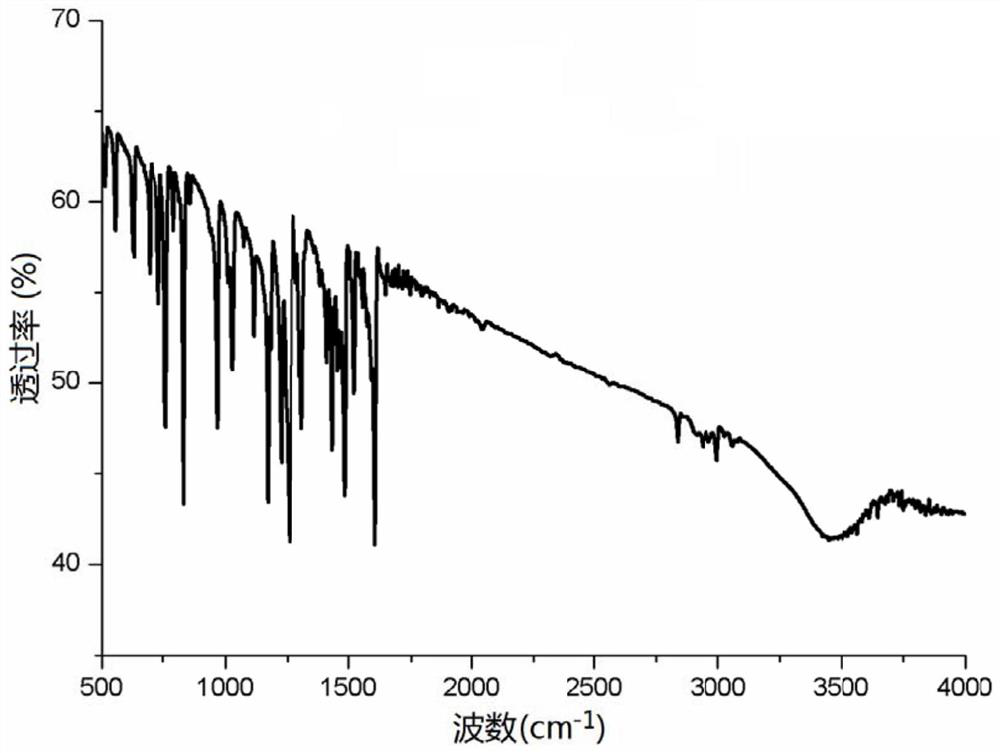
[0031] Example 1: Divalent palladium imine complex catalyzes the hydroamination of cyclohexylacetylene to prepare enamine
[0032]
[0033] A method for preparing enamine compounds by catalyzing cyclohexylacetylene hydroamination reaction, the specific steps are:
[0034] Catalyst [CH shown in formula (I) 2 (C 5 h 4 N) 2 ]Pd=NPh (3.7mg, 0.00001mol), cyclohexylacetylene (1.08g, 0.01mol) and methylphenylamine (1.02g, 0.01mol) and 6mL toluene were added to the reaction tube, under the catalysis of the catalyst, the ring Hydroamination reaction of hexylacetylene and secondary amine in toluene, the reaction temperature is 60°C, the reaction time is 8h, after the end, extract the system with 20mL ethyl acetate and 10mL water, separate the liquid, add 5mL ethyl acetate to the obtained organic phase Extract with 5mL water twice, separate liquid, combine organic phase, carry out drying with anhydrous sodium sulfate, then filter and concentrate, reaction solution is through silic...
Embodiment 2
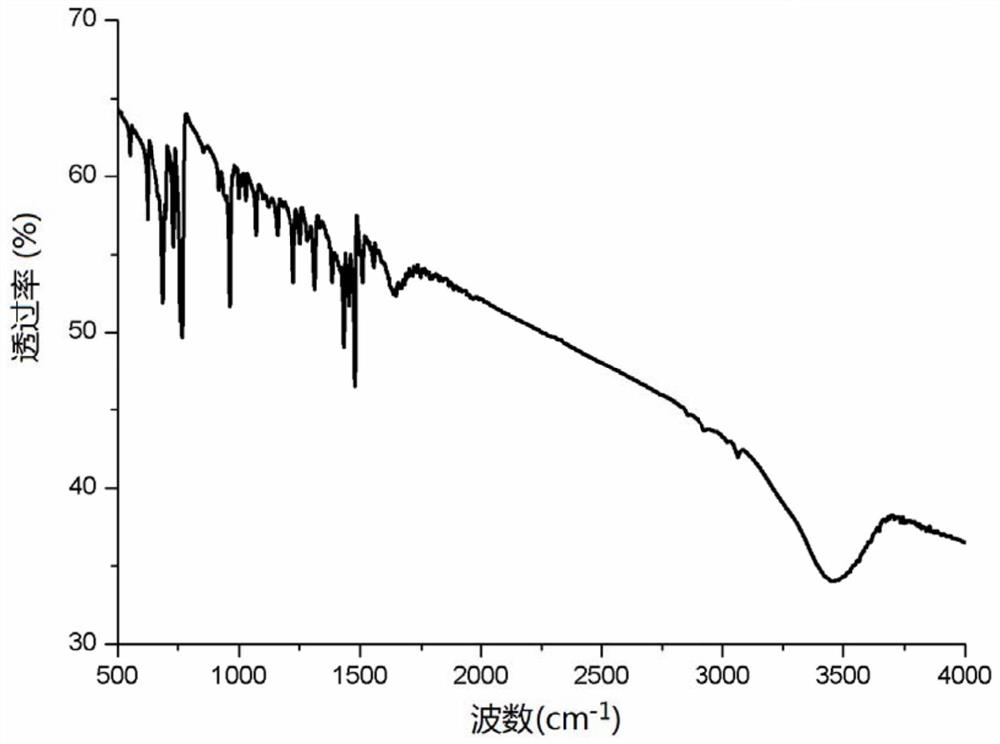
[0035] Example 2: Divalent palladium imine complex catalyzes the hydroamination of cyclohexylacetylene to prepare enamine
[0036]
[0037] A method for preparing enamine compounds by catalyzing cyclohexylacetylene hydroamination reaction, the specific steps are:
[0038] Catalyst [CH shown in formula (I) 2 (C 5 h 4 N) 2 ]Pd=NPh (3.7mg, 0.00001mol), cyclohexylacetylene (1.29g, 0.012mol) and methylethylamine (1.02g, 0.01mol) and 8mL toluene were added to the reaction tube, under the catalysis of the catalyst, the ring Hexylacetylene and secondary amine undergo hydroamination reaction in toluene, the reaction temperature is 75°C, and the reaction time is 6h. After the end, the system is extracted with 20mL ethyl acetate and 10mL water, and the obtained organic phase is added with 5mL ethyl acetate Extract with 5mL water twice, separate liquid, combine organic phase, carry out drying with anhydrous sodium sulfate, then filter and concentrate, reaction solution is through s...
Embodiment 3
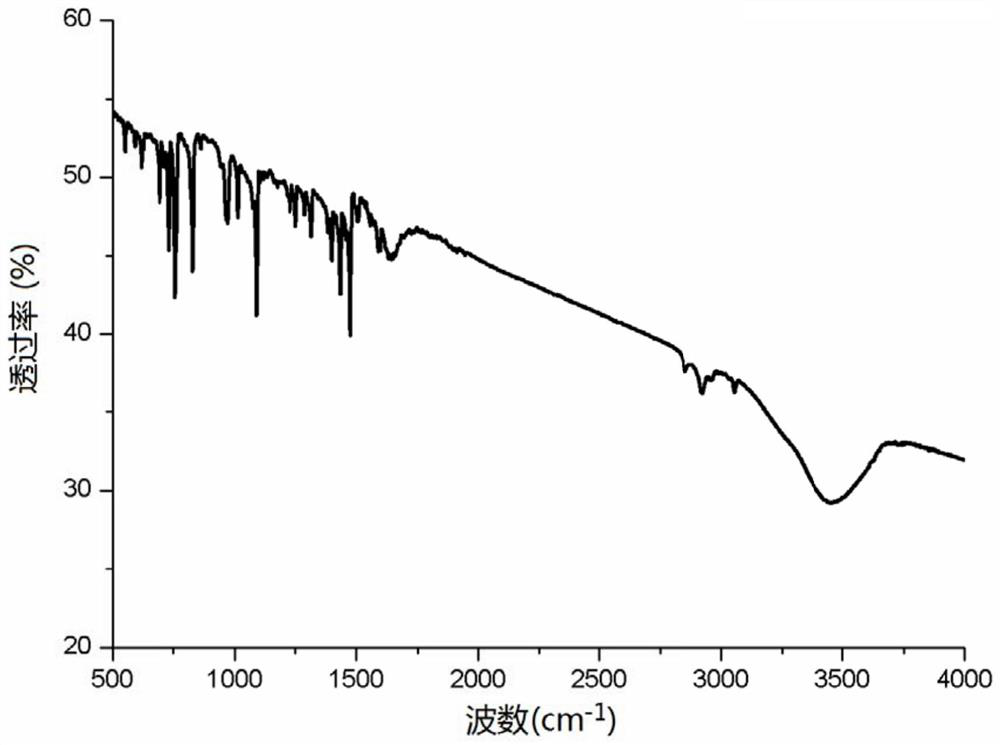
[0039] Example 3: Divalent palladium imine complex catalyzes the hydroamination of cyclohexylacetylene to prepare enamine
[0040]
[0041] A method for preparing enamine compounds by catalyzing cyclohexylacetylene hydroamination reaction, the specific steps are:
[0042] The catalyst shown in formula (I) [(CH 3 )CH(C 5 h 4 N) 2 ]Pd=NPh (7.6mg, 0.00002mol), cyclohexylacetylene (1.62g, 0.015mol) and methyl isopropylamine (1.02g, 0.01mol) and 10mL toluene were added to the reaction tube, under the catalysis of the catalyst, Cyclohexylacetylene and secondary amines undergo hydroamination reaction in toluene, the reaction temperature is 100°C, and the reaction time is 12h. After the completion, the system is extracted with 20mL ethyl acetate and 10mL water, and the obtained organic phase is added with 5mL ethyl acetate The ester was extracted twice with 5 mL of water, separated, and the organic phases were combined, dried with anhydrous sodium sulfate, filtered and concentr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com