Method for catalytically synthesizing aryl oxazole compound by using nickel complex
A technology of nickel complexes and aryl oxazoles, which is applied in nickel organic compounds, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., and can solve problems such as high reaction temperature and large catalyst equivalent , to achieve the effects of high product yield, stable physical and chemical properties, and high substrate universality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
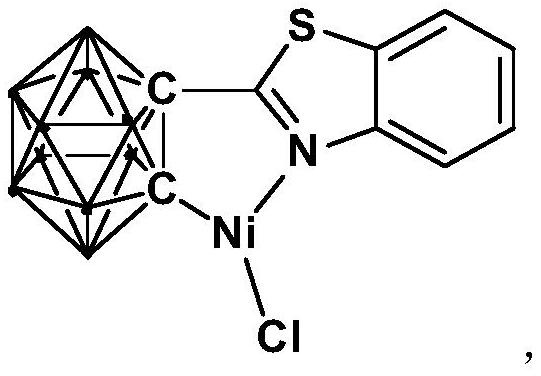
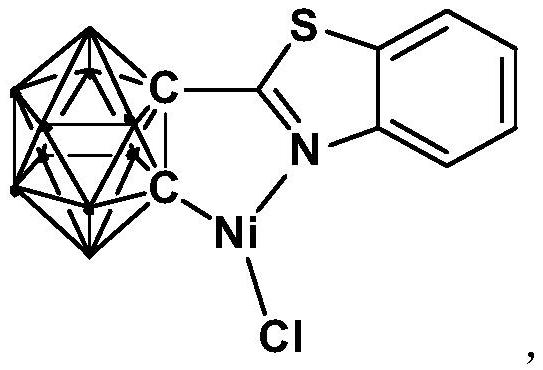
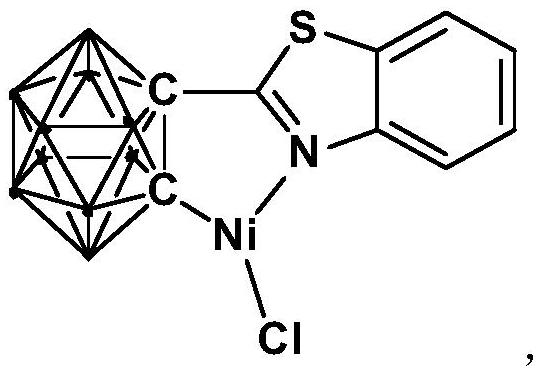
[0031] The preparation method of the nickel complex containing the ortho carboryl benzothiazole structure comprises the following steps:
[0032] 1) Add the n-BuLi solution to the ortho-carborane solution at -80°C to -75°C, stir and react at room temperature for 30-60min;
[0033] 2) Add bromobenzothiazole and react at room temperature for 6-8h;
[0034] 3) Add NiCl 2 , and react at room temperature for 3-5h, after post-treatment to obtain nickel complexes.
[0035] In step 1), the n-BuLi solution is n-BuLi n-hexane solution, and the ortho carborane solution is ortho carborane tetrahydrofuran solution.
[0036] In step 3), the post-treatment process is: standing and filtering after the reaction, decompressing to dry the solvent to obtain the crude product, and then subjecting the crude product to column chromatography separation; during the column chromatography separation process, the eluent is petroleum ether and A mixed solvent of tetrahydrofuran, and the volume ratio of...
Embodiment 1
[0039] Synthesis of nickel complexes containing ortho carboryl benzothiazole structure:
[0040] At -78°C, n-BuLi n-hexane solution (1.6 mmol) was slowly added dropwise to the ortho-position carborane o-C 2 B 10 h 12 (0.64mmol) in tetrahydrofuran solution, stirred at this temperature for 30 minutes, slowly rose to room temperature and continued to react for 1 hour, then added bromobenzothiazole (0.64mmol), and continued to react at room temperature for 6 hours. Then NiCl 2 (0.64 mmol) was added to the reaction system and reacted for another 3 hours. After the reaction, stand and filter, and dry the solvent under reduced pressure. The obtained crude product is separated by column chromatography (by volume, petroleum ether / tetrahydrofuran=6:1), and the brown target product is obtained, which is the ortho carbon-containing The nickel complex (yield 78%) of boryl benzothiazole structure, the reaction formula is:
[0041]
[0042] Among them, "·" represents the boron-hydrog...
Embodiment 2
[0045] Nickel complexes catalyze the synthesis of aryl oxazole compounds, the specific process is as follows:
[0046]Using the nickel complex prepared in Example 1 as a catalyst, the nickel complex (0.001mmol), benzoxazole (1.0mmol), chlorobenzene (1.1mmol) and potassium carbonate (1.2mmol) were dissolved in 2 mL of toluene, and reacted at room temperature After 100 minutes, after the end, the concentrated reaction solution was directly separated by silica gel column chromatography, dried until the mass remained constant, and the corresponding product C was obtained. 13 h 9 NO (90% yield), the reaction formula is:
[0047]
[0048] 1 H NMR (400MHz, CDCl 3 )δ: 8.12(d, J=8.0Hz, 2H), 7.44(s, 1H), 7.36(d, J=8.0Hz, 1H), 7.10-7.01(m, 5H). HRMS theoretical value C 13 h 9 NO(M) + : 195.0684, actual measured value: 195.0688.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com