N,N-coordinated palladium complex with meta-carborane ligand and preparation and application of palladium complex
A technology of m-carborane and palladium complexes, applied in the field of synthetic chemistry, can solve problems such as mild reaction conditions, small substrate adaptability, use of toxic reagents, etc., and achieve easy separation, low-cost, low-toxicity separation, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
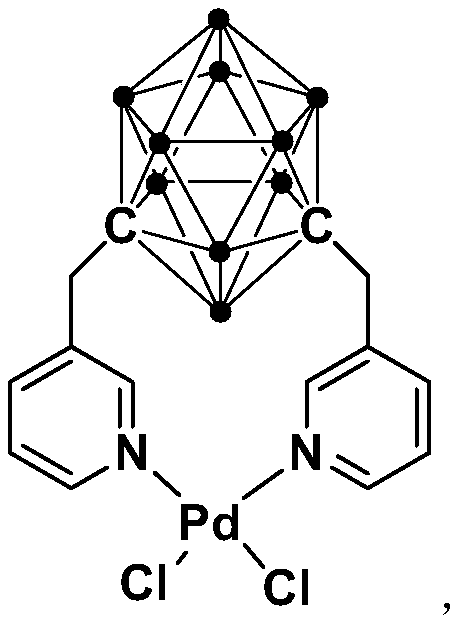
[0032] Synthesis of N,N-coordinated divalent palladium complexes Pd containing m-carborane ligands:
[0033]
[0034] At -78°C, n-BuLi (1.6M) in n-hexane (1.00 mL, 1.6 mmol) was slowly added dropwise to m-C containing ortho carborane 2 B 10 h 10 (92.0mg, 0.64mmol) in tetrahydrofuran solution, stirred at this temperature for 30 minutes, slowly rose to room temperature and continued to react for 1 hour, then added 3-chloromethylpyridine (162.3mg, 1.28mmol), continued to react at room temperature 5 hours. Then PdCl 2 (112.0 mg, 0.64 mmol) was added to the reaction system for an additional 2 hours. After the reaction is over, let it sit and filter, and dry the solvent under reduced pressure. The obtained crude product is separated by column chromatography (petroleum ether / ethyl acetate=8:1), and the brown target product palladium complex Pd (231.8 mg, produced rate of 72%).
[0035] 1 H NMR (400MHz, CDCl 3 ,25℃):δ=7.89(d,J=7.0Hz,2H),7.58(s,2H),7.42(d,J=6.5Hz,2H),7.30(t,...
Embodiment 2
[0038] Palladium complexes catalyze the formylation of anilines:
[0039]
[0040] The palladium complex Pd prepared in Example 1 was used as a catalyst to catalyze the formylation of aniline: aniline (1mmol, 93mg) and divalent palladium complex Pd (0.01mmol, 5.0mg) were dissolved in 4mL DMF, at 60°C The reaction was carried out for 6 hours. After the end, the concentrated reaction solution was directly separated by silica gel column chromatography, and dried until the quality remained unchanged to obtain the corresponding product C 7 h 7 NO (92% yield), 1 H NMR (400MHz, CDCl 3 , 25°C): δ=9.82(s, 1H), 7.76(d, J=7.2Hz, 2H), 7.60-752(m, 3H), 6.52(s, 1H). Elemental analysis: C 69.41, H 5.82, N 11.56 (theoretical); C 69.51, H 5.88, N 11.49 (actual).
Embodiment 3
[0042] Palladium complexes catalyze the formylation of 4-methylanilide:
[0043]
[0044] The palladium complex Pd prepared in Example 1 was used as a catalyst to catalyze the formyl reaction of 4-methylaniline: 4-methylaniline (1mmol, 107mg) and divalent palladium complex Pd (0.002mmol, 1.0mg) were dissolved In 4mL DMF, react at 90°C for 8 hours. After the end, the concentrated reaction solution is directly separated by silica gel column chromatography, dried until the mass remains constant, and the corresponding product C is obtained. 8h 9 NO (82% yield), 1 H NMR (400MHz, CDCl 3 ,25℃):δ=9.85(s,1H),7.79(d,J=7.2Hz,2H),7.52(d,J=7.2Hz,2H),6.57(s,1H),2.23(s,3H ). Elemental analysis: C 71.09, H 6.71, N 10.36 (theoretical); C71.01, H 6.78, N 10.40 (actual).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com