Preparation and application of a large sterically hindered trivalent rhodium imine complex
A technology of large steric hindrance and rhodium imine, applied in the preparation of organic compounds, preparation of amino hydroxyl compounds, rhodium organic compounds, etc., to achieve the effect of high atom economy, simple preparation method and stable physical and chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
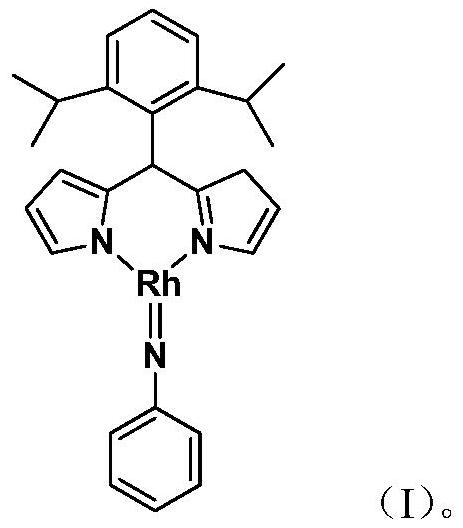
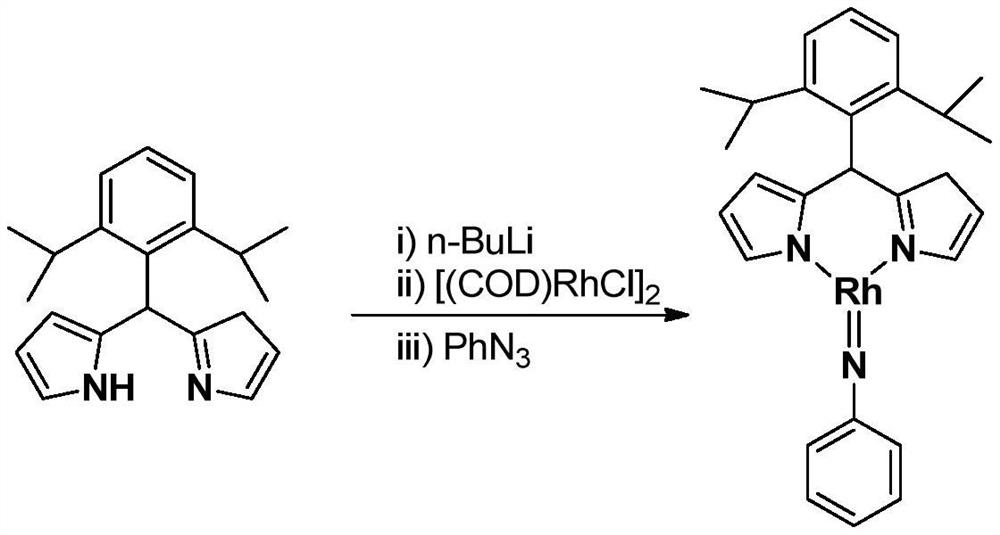
[0024] Embodiment 1: the synthesis of the trivalent rhodium imine complex of large steric hindrance containing rhodium-nitrogen double bond structure
[0025]
[0026] At –78°C, n-BuLi (1.6M) in n-hexane (0.50 mL, 0.8 mmol) was slowly added dropwise to 5 mL of diisopropyl-substituted phenyldipyrrole C 21 h 26 N 2 (183.0mg, 0.60mmol) in tetrahydrofuran solution, continue to stir at this temperature for 1 hour after the dropwise addition, slowly rise to room temperature and continue to react for 1 hour, then add the monovalent rhodium precursor cyclooctadiene rhodium chloride dimerization Body [(COD)RhCl] 2 (148.0mg, 0.30mmol), continue to react at room temperature for 3 hours. Then the phenylazide PhN 3 (107.0mg, 0.90mmol) was added to the reaction system and reacted at room temperature for 3 hours. After the reaction, stand and filter, and dry the solvent under reduced pressure. The obtained crude product is separated by silica gel column chromatography (eluent: petrol...
Embodiment 2
[0028] Embodiment 2: trivalent rhodium imine complexes catalyze the anti-Martensoyl hydroamination reaction of olefins
[0029]
[0030] The catalyst prepared in Example 1 was used to catalyze the reverse Martensohydroamination reaction of olefins: 5 mL of imine complex containing trivalent rhodium (0.001 mmol, 5.0 mg) was added to styrene (1 mmol, 104 mg) and aniline (1 mmol, 93 mg). ) in toluene solution, the reaction temperature is 25°C, and the reaction time is 70 minutes. After the end, the reaction solution is concentrated, and directly separated by silica gel column chromatography (eluent: petroleum ether / ethyl acetate (v / v)=8:1) , dried until the mass remains unchanged, and the corresponding amine compound C 14 h 15 N (185mg, yield 88%), 1 H NMR (400MHz, CDCl 3 ,25℃):δ=7.40-7.32(m,2H),7.28-7.20(m,5H),6.81-6.74(m,1H),6.70(d,2H),3.75(br s,1H),3.45 (t, J=6.8Hz, 2H), 2.99 (t, J=6.8Hz, 2H); elemental analysis: C 85.24, H 7.66, N 7.10 (theoretical); C 85.26, H 7.61, N...
Embodiment 3
[0031] Embodiment 3: trivalent rhodium imine complexes catalyze the anti-Martensitic hydroamination reaction of olefins
[0032]
[0033] The catalyst prepared in Example 1 was used to catalyze the reverse Martensohydroamination reaction of olefins: 5 mL of imine complex containing trivalent rhodium (0.002 mmol, 10.0 mg) was added to styrene (1 mmol, 104 mg) and aniline (1 mmol, 93 mg). ) in toluene solution, the reaction temperature is 25°C, and the reaction time is 60 minutes. After the completion, the concentrated reaction solution is directly separated by silica gel column chromatography (eluent: petroleum ether / ethyl acetate (v / v)=8:1), Dry until the mass remains constant to obtain the corresponding amine compound C 15 h 17N (192 mg, yield 91%), 1 H NMR (400MHz, CDCl 3 ,25℃):δ=7.42-7.33(m,1H),7.29-7.23(m,5H),6.80-6.74(m,1H),6.65(d,2H),3.75(br s,1H),3.45 (t, J = 6.8Hz, 2H), 2.99 (t, J = 6.8Hz, 2H), 2.32 (s, 3H); elemental analysis: C 85.26, H 8.11, N 6.63 (theoretic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com