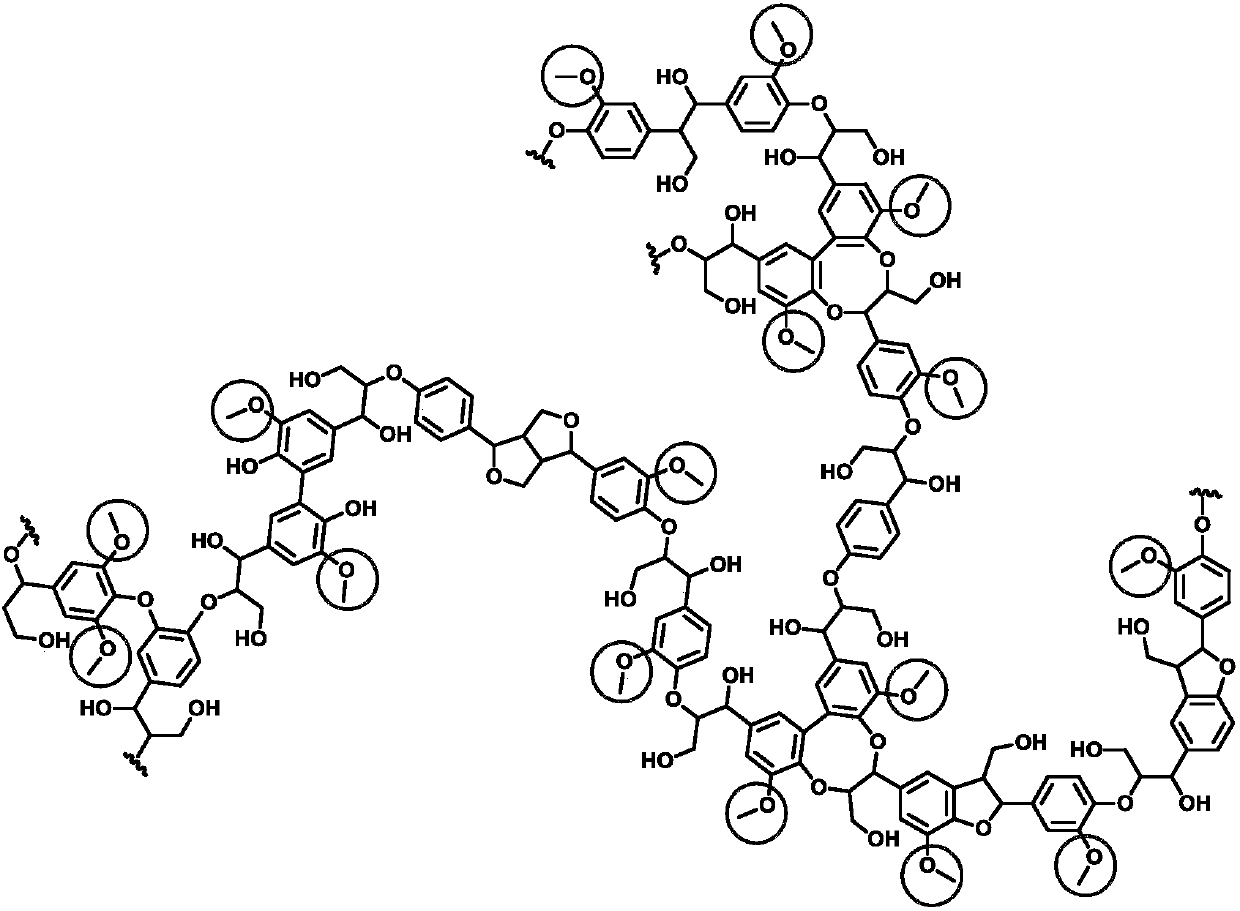
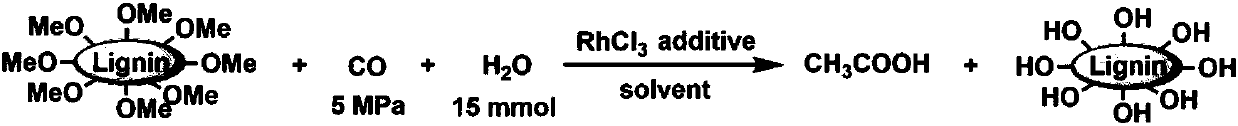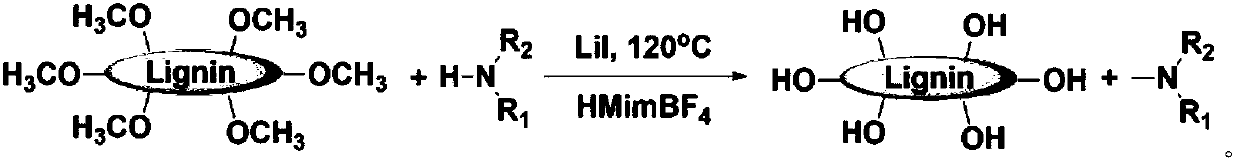Method for preparing important chemicals by using lignin as methyl sources
A lignin and methyl source technology, applied in chemical instruments and methods, amino compound preparation, bulk chemical production, etc., can solve problems such as low conversion rate, difficult reaction and utilization, complex structure, etc., and achieve important application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the preparation of acetic acid
[0041] Lignin was used as a methyl source to react with carbon monoxide and water to prepare acetic acid in a stainless steel reactor with a polytetrafluoroethylene liner with a volume of 16 ml, and the reactor was stirred by a magnet. In the experiment, a certain amount of ruthenium or rhodium-containing catalyst, iodide and fluoroborate additives, lignin, water and reaction solvent are added to the reactor, and the reactor is sealed and replaced three times with 1MPa nitrogen gas. Carbon monoxide at a specific pressure is charged into the reactor. The reactor was placed in an air bath at a constant temperature, and the electromagnetic stirring speed was set at 800 rpm. After the reaction, the reactor was cooled in an ice-water bath, and the gas was released. About 0.04 g of 1,3,5-trioxane was added as an internal standard to the reacted liquid mixture, and then the liquid mixture was diluted with 10 ml of methanol and s...
Embodiment 2
[0097] Embodiment 2: the preparation of N-methylation product
[0098] Lignin was used as a methyl source to react with amine compounds to prepare N-methyl compounds in a 16ml stainless steel reactor with a polytetrafluoroethylene liner, and the reactor was magnetically stirred. In the experiment, a certain amount of iodide catalyst and optional fluoroborate co-catalyst, lignin or anisole, amine and reaction solvent are added to the reactor, the reactor is sealed and replaced three times with 1MPa nitrogen, and The reaction kettle was placed in an air bath at a constant temperature, and the electromagnetic stirring speed was set at 800 rpm. After the reaction, the reactor was cooled in an ice-water bath. About 0.04 g of 1,3,5-trioxane was added as an internal standard to the reacted liquid mixture, and then the liquid mixture was diluted with 10 ml of methanol and stirred for 5 minutes. The above mixture was centrifuged with 1 H NMR (Bruker Avance III 400HD), the deuterium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com