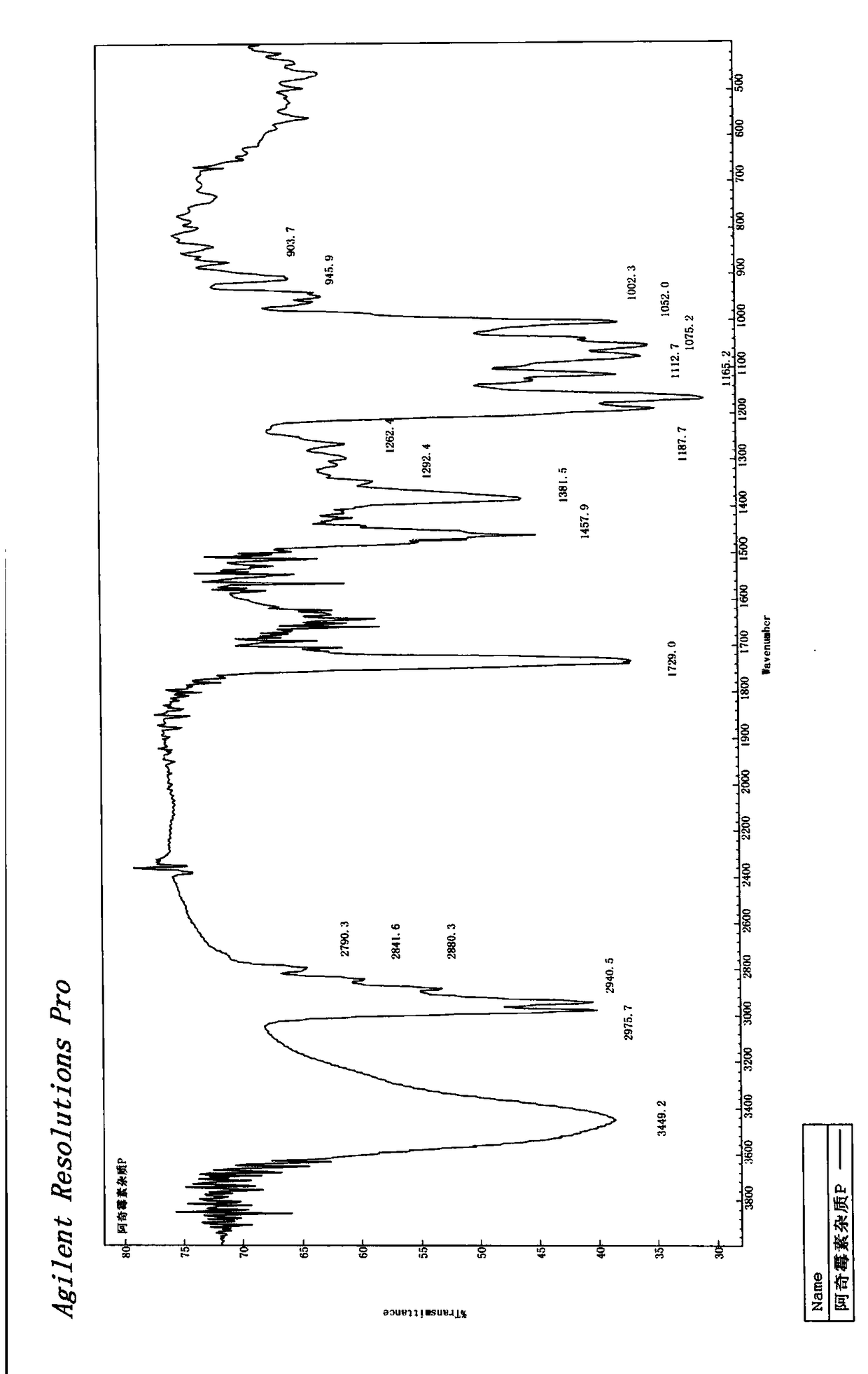
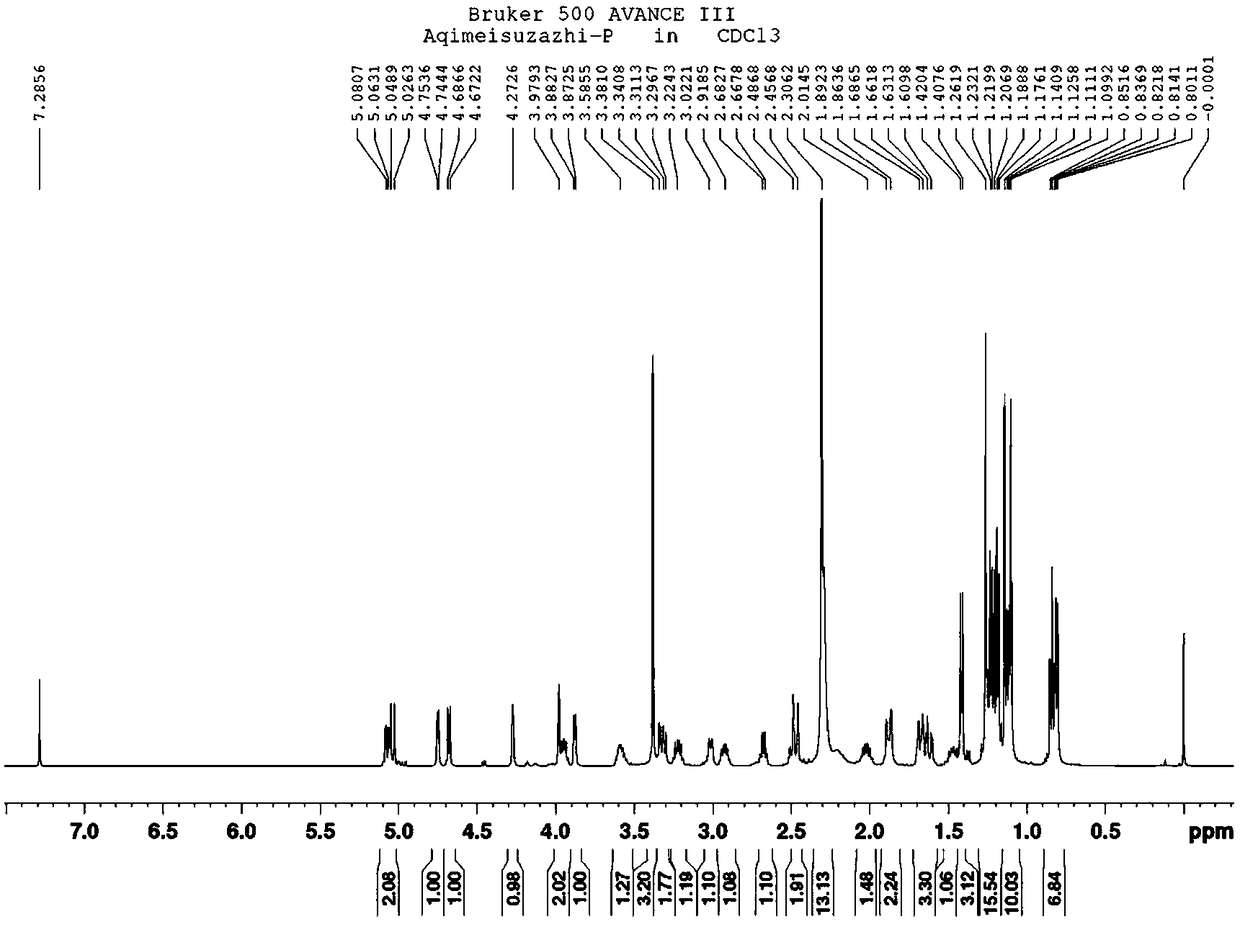
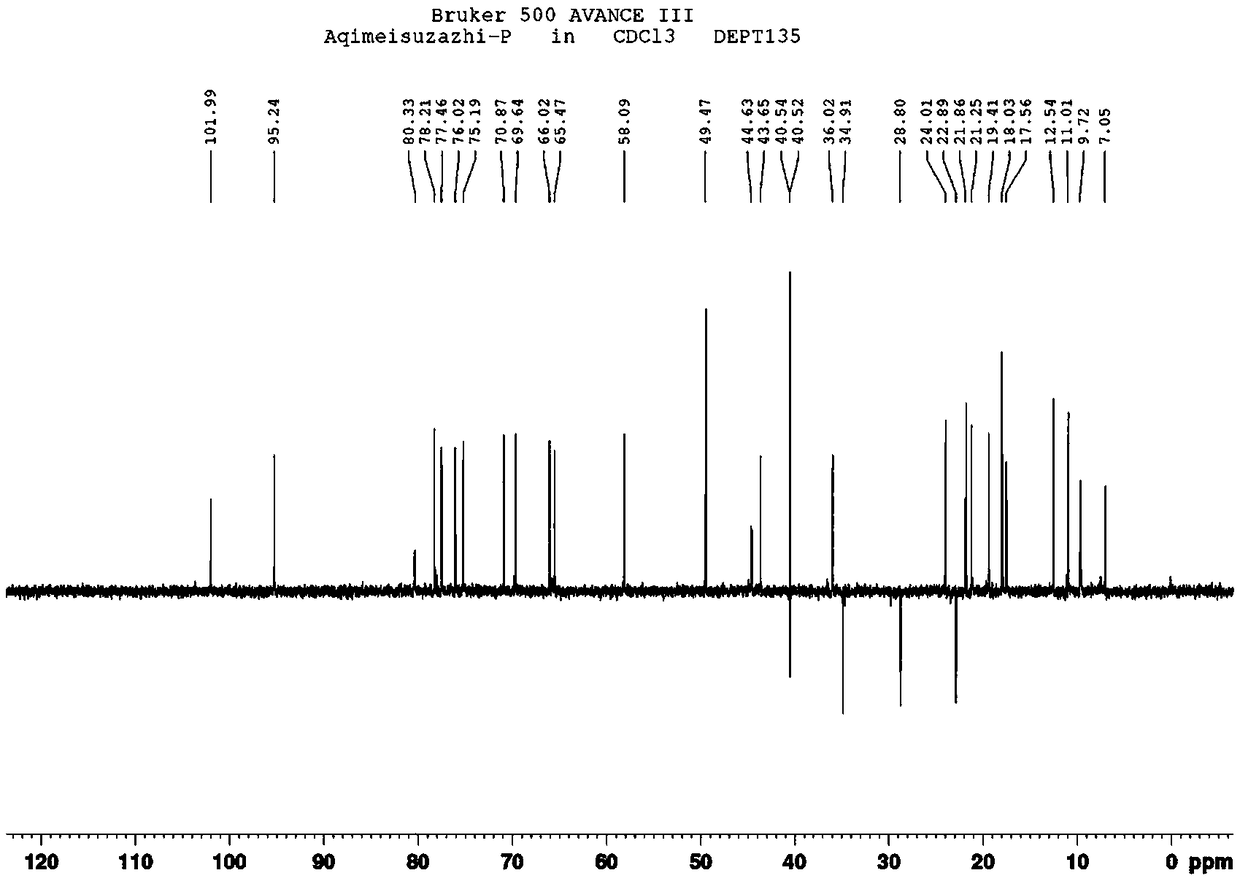
Azithromycin related substance and preparation method thereof
A technology related to substances and azithromycin, applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Pretreatment of platinum carbon
[0033] Method 1: Take 50 g of platinum carbon (platinum content 5%), add 300 ml of water, stir, and add 0.72 g of ferric chloride, beat for 0.5 hours, and filter to obtain the treated platinum carbon catalyst.
[0034] Method 2: Take 50 g of platinum carbon (platinum content 5%), add 300 ml of water, stir, and add 0.69 g of calcium chloride, beat for 0.5 hour, and filter to obtain the treated platinum carbon catalyst.
[0035] Method 3: Take 50 g of platinum carbon (platinum content 5%), add 300 ml of water, stir, and add 0.53 g of copper chloride, beat for 0.5 hours, and filter to obtain the treated platinum carbon catalyst.
Embodiment 2
[0038] Take 30 g of erythromycin 6,9-imine ether, add 200 ml of methanol, stir to dissolve, and adjust the pH to 4 to 5 with hydrochloric acid. The platinum carbon treated according to method 1 in Example 1 was put into the autoclave, and then the dissolved material was put into the autoclave together to start the hydrogenation operation. The hydrogen pressure is maintained between 1.0-1.2 MPa, the temperature is slowly raised to 55-60°C, and the reaction is carried out for 8-12 hours. After the reaction is over, the hydrogenation feed liquid is taken out, the catalyst is removed by filtration, and the feed liquid is recovered and dried. Add 100ml of acetone to dissolve, add 10g of formic acid, 15g of formaldehyde (37% aqueous solution), adjust the pH to 5-6 with sodium hydroxide (30% aqueous solution), and keep it at 40-50°C for 20-30 hours. After the reaction, the pH value is adjusted to 10-11 with sodium hydroxide (30% aqueous solution). Let stand for layering, take the ac...
Embodiment 3
[0040] Take 30 g of erythromycin 6,9-imine ether, add 200 ml of methanol, stir to dissolve, and adjust the pH to 4 to 5 with hydrochloric acid. Put the platinum carbon treated according to method 2 in Example 1 into the autoclave, and then put the dissolved material into the autoclave together to start the hydrogenation operation. The hydrogen pressure is maintained between 1.0-1.2 MPa, the temperature is slowly raised to 55-60°C, and the reaction is carried out for 8-12 hours. After the reaction is over, the hydrogenation feed liquid is taken out, the catalyst is removed by filtration, and the feed liquid is recovered and dried. Add 100ml of acetone to dissolve, add 10g of formic acid, 15g of formaldehyde (37% aqueous solution), adjust the pH to 5-6 with sodium hydroxide (30% aqueous solution), and keep it at 40-50°C for 20-30 hours. After the reaction, the pH value is adjusted to 10-11 with sodium hydroxide (30% aqueous solution). Let stand for layering, take the acetone or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com