N-methylation method of aromatic amine
A technology for the methylation of aromatic amines, applied in the field of methylation of amines, can solve the problems of poor reactivity and low yield of N-monomethyl products, and achieve the effect of simple operation and wide application range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
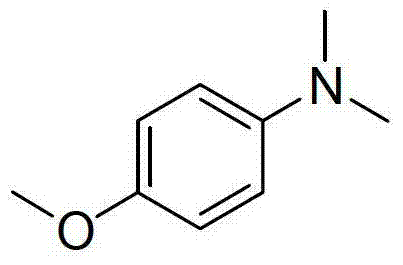
[0013] Preparation of N,N-dimethyl-p-methoxyaniline with the following structural formula
[0014]
[0015] Add 123mg (1mmol) of p-methoxyaniline, 3mL of dimethyl sulfoxide, 0.75mL (20mmol) of formic acid, and 2.78mL (20mmol) of triethylamine into a thick-walled pressure-resistant tube. Gas, sealed thick-walled pressure tube, stirred at 150°C for 12 hours, cooled to room temperature, adjusted the pH value of the reaction solution to alkaline with saturated aqueous sodium hydroxide solution, extracted with dichloromethane (5×3mL), and used Dry over sodium sulfate, remove dichloromethane by distillation under reduced pressure, use a mixture of petroleum ether and ethyl acetate with a volume ratio of 50:1 as eluent, separate the product by flash column chromatography, and prepare N,N-dichloromethane Methyl p-methoxyaniline, its productive rate is 92%, and the characterization data of product are: 1 H NMR (400MHz, CDCl 3 )δ(ppm):6.85(d,J=9.1Hz,2H),6.76(d,J=9.1Hz,2H),3.77(s,3H...
Embodiment 2
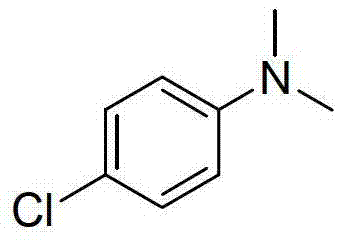
[0017] Preparation of N,N-dimethyl-p-chloroaniline with the following structural formula
[0018]
[0019] Add 127mg (1mmol) of p-chloroaniline, 3mL of dimethyl sulfoxide, 0.68mL (18mmol) of formic acid, and 2.50mL (18mmol) of triethylamine into a thick-walled pressure-resistant tube, and bubble with argon for 15 minutes, blowing argon, Seal the thick-walled pressure-resistant tube, stir at 150°C for 12 hours, cool to room temperature, adjust the pH value of the reaction solution to alkaline with saturated aqueous sodium hydroxide solution, extract with dichloromethane (5×3mL), and use anhydrous sulfuric acid for the organic phase Sodium drying, vacuum distillation to remove dichloromethane, the volume ratio of petroleum ether and ethyl acetate is 400:1 mixed solution as eluent, flash column chromatography to separate the product, and prepare N,N-dimethyl p-Chloroaniline, its productive rate is 89%, and the characterization data of product are: 1 H NMR (400MHz, CDCl 3 )δ(...
Embodiment 3
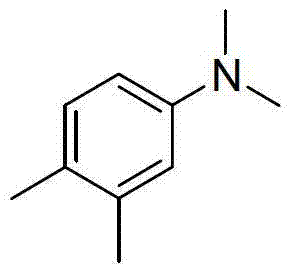
[0021] Preparation of N,N-dimethyl-3,4-dimethylaniline with the following structural formula
[0022]
[0023] Add 121mg (1mmol) of 3,4-dimethylaniline, 3mL of dimethyl sulfoxide, 0.83mL (22mmol) of formic acid, and 3.06mL (22mmol) of triethylamine into a thick-walled pressure-resistant tube, and bubble with argon for 15 minutes , blow argon, seal the thick-walled pressure tube, stir at 140°C for 24 hours, cool to room temperature, adjust the pH value of the reaction solution to alkaline with saturated aqueous sodium hydroxide solution, extract with dichloromethane (5×3mL), organic The phase was dried with anhydrous sodium sulfate, dichloromethane was removed by distillation under reduced pressure, and the mixed solution with a volume ratio of petroleum ether and ethyl acetate of 300:1 was used as an eluent, and the product was separated by flash column chromatography to prepare N, N-dimethyl-3,4-dimethylaniline, its productive rate is 88%, and the characteristic data of pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com