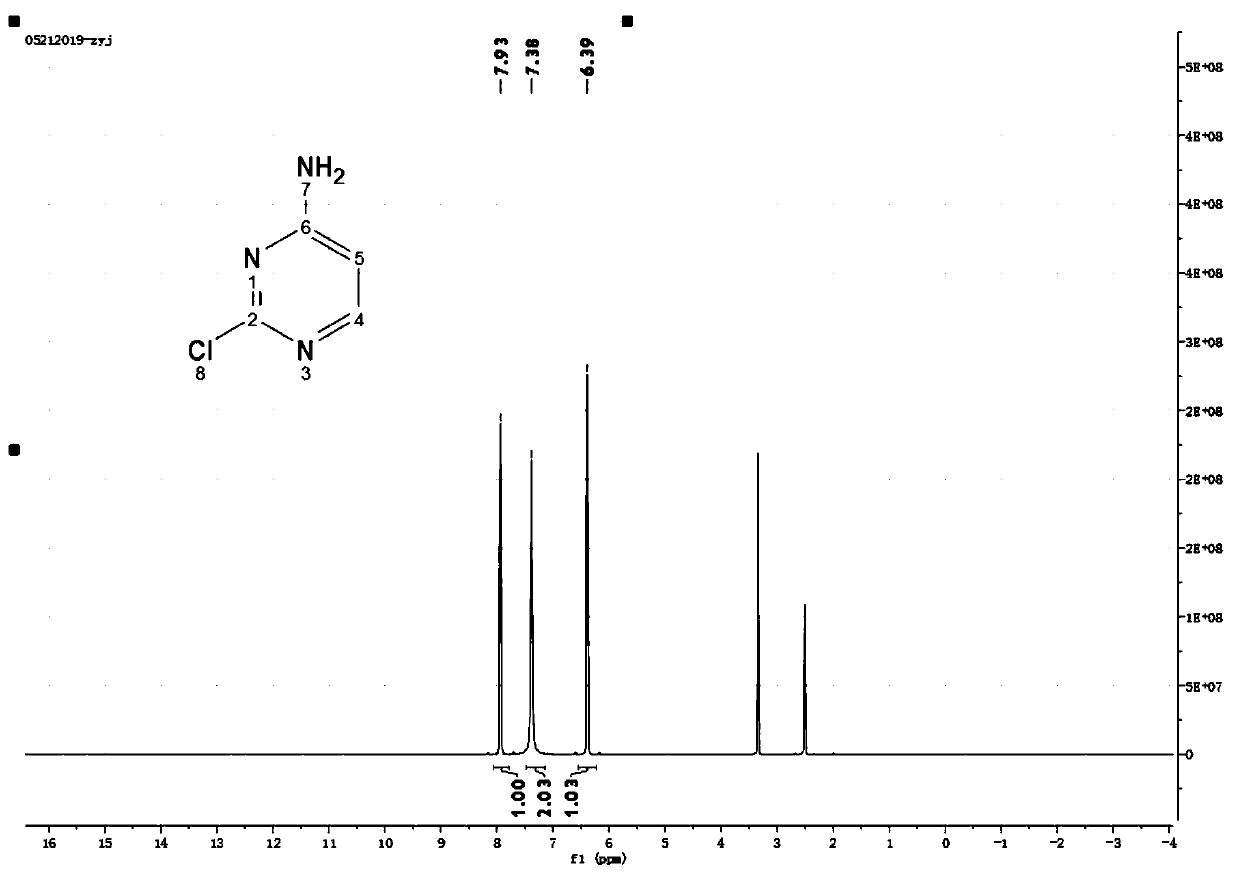
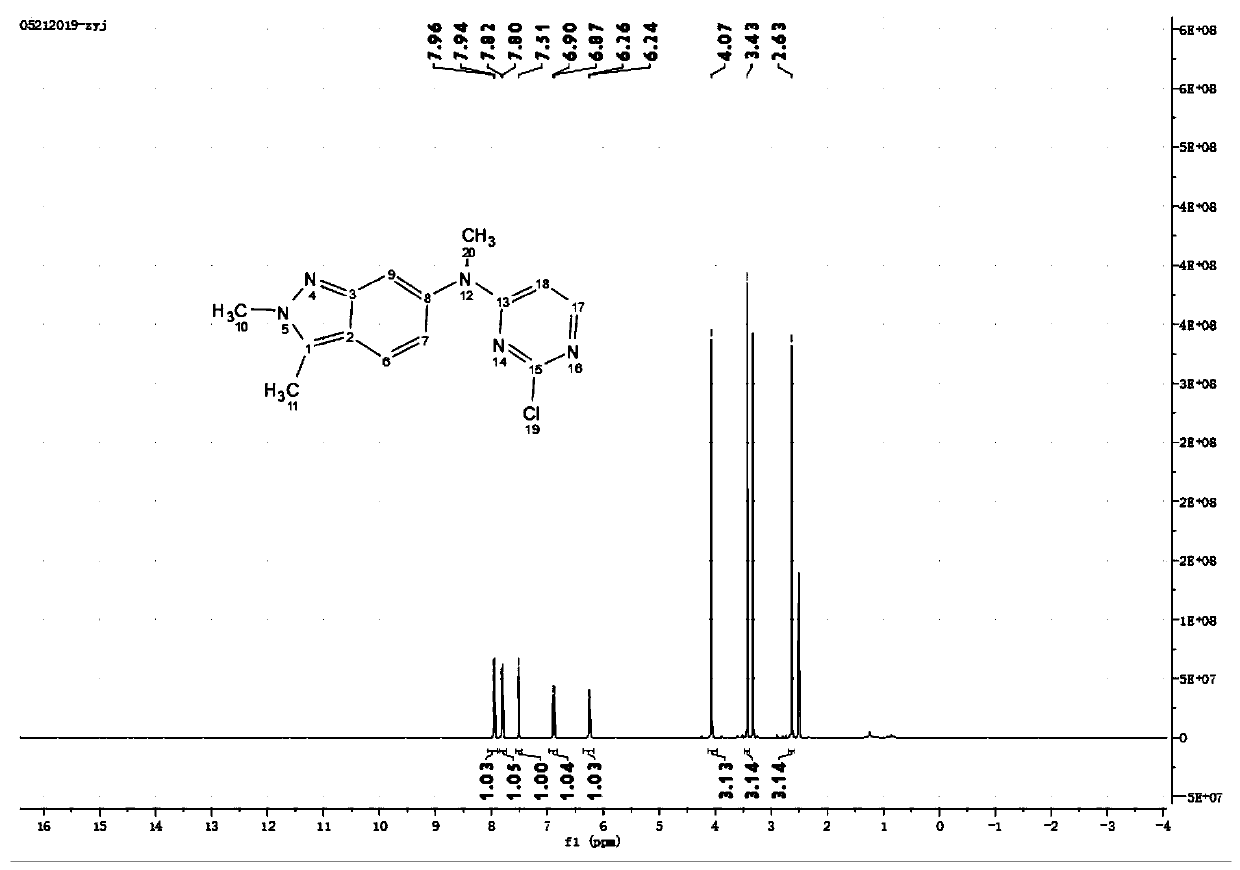
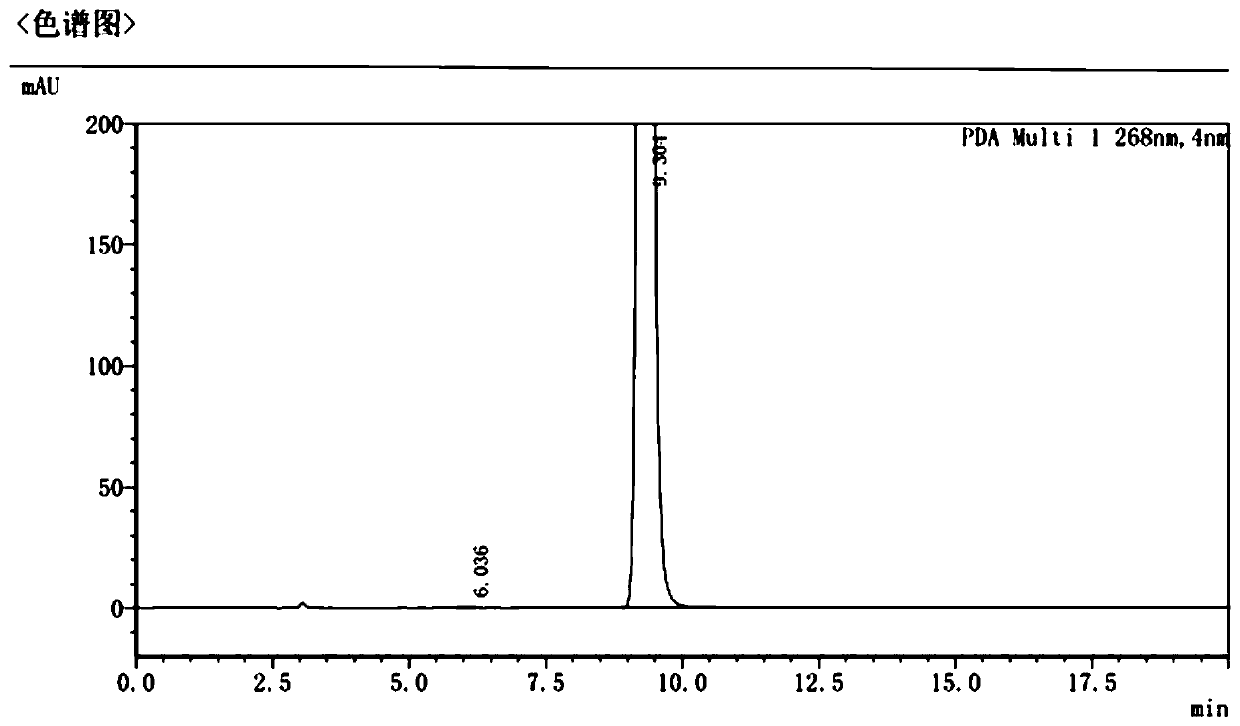
Green preparation method of pazopanib hydrochloride
A technology of pazopanib hydrochloride and indole hydrochloride, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of unfavorable production cost, complicated waste acid solution, and high environmental protection pressure, so as to avoid by-products and improve the reaction yield High, risk-reducing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] Add 11g of o-methylaniline and 80mL of dichloromethane into a closed reaction flask, stir evenly and dissolve completely, add 15.2g of N-chlorosuccinimide in batches; after the addition, the mixture is slowly heated to reflux, and the The reaction was stirred for 7 hours in the state, and the reaction of the raw materials was detected by gas chromatography (GC). The reaction mixture was concentrated in vacuo, and the concentrate was added to 100 mL of saturated sodium chloride solution. After stirring for 30 minutes, it was allowed to stand for 10 minutes, and then 50 mL of ethyl acetate was added to extract The reaction solution was repeated several times, and the combined organic phases were washed with brine, dried over anhydrous sodium sulfate, and concentrated in vacuo to obtain 10.5 g of 2-methyl-5-chloro-aniline, whose purity was 98.4% as detected by GC.
Embodiment 2
[0042]
[0043] Add 11g of o-methylaniline and 80mL of dichloromethane into a closed reaction flask, stir evenly and completely dissolve, add 21g of N-chlorosuccinimide in batches; after the addition, slowly heat the mixture to reflux, in this state The reaction was stirred for 7 hours, and the reaction of the raw materials was detected by gas chromatography (GC). The reaction mixture was concentrated in vacuo, and the concentrate was added to 100 mL of saturated sodium chloride solution. After stirring for 30 minutes, it was allowed to stand for 10 minutes, and then 50 mL of ethyl acetate was added to extract the reaction. solution several times, the combined organic phase was washed with brine, dried with anhydrous sodium sulfate, and concentrated in vacuo to obtain 12.1 g of 2-methyl-5-chloro-aniline, and the purity of the product detected by GC was 95.9%, and the impurities were mainly Multiple substitution by-products.
Embodiment 3
[0045]
[0046] Add 11g of o-methylaniline and 80mL of dichloromethane into a closed reaction flask, stir evenly and dissolve completely, add 11.2g of N-chlorosuccinimide in batches; after the addition, the mixture is slowly heated to reflux, and the The reaction mixture was stirred and reacted for 7 hours under the state, the reaction mixture was concentrated in vacuo, the concentrate was added to 100mL saturated sodium chloride solution, stirred for 30min and then allowed to stand for 10min, then 50mL ethyl acetate was added to extract the reaction solution several times, and the combined organic phase was used It was washed with brine, dried over anhydrous sodium sulfate, and concentrated in vacuo to obtain 7.8 g of 2-methyl-5-chloro-aniline. The purity of the product was 92.7% as detected by GC, and the impurities were mainly unreacted raw materials.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com