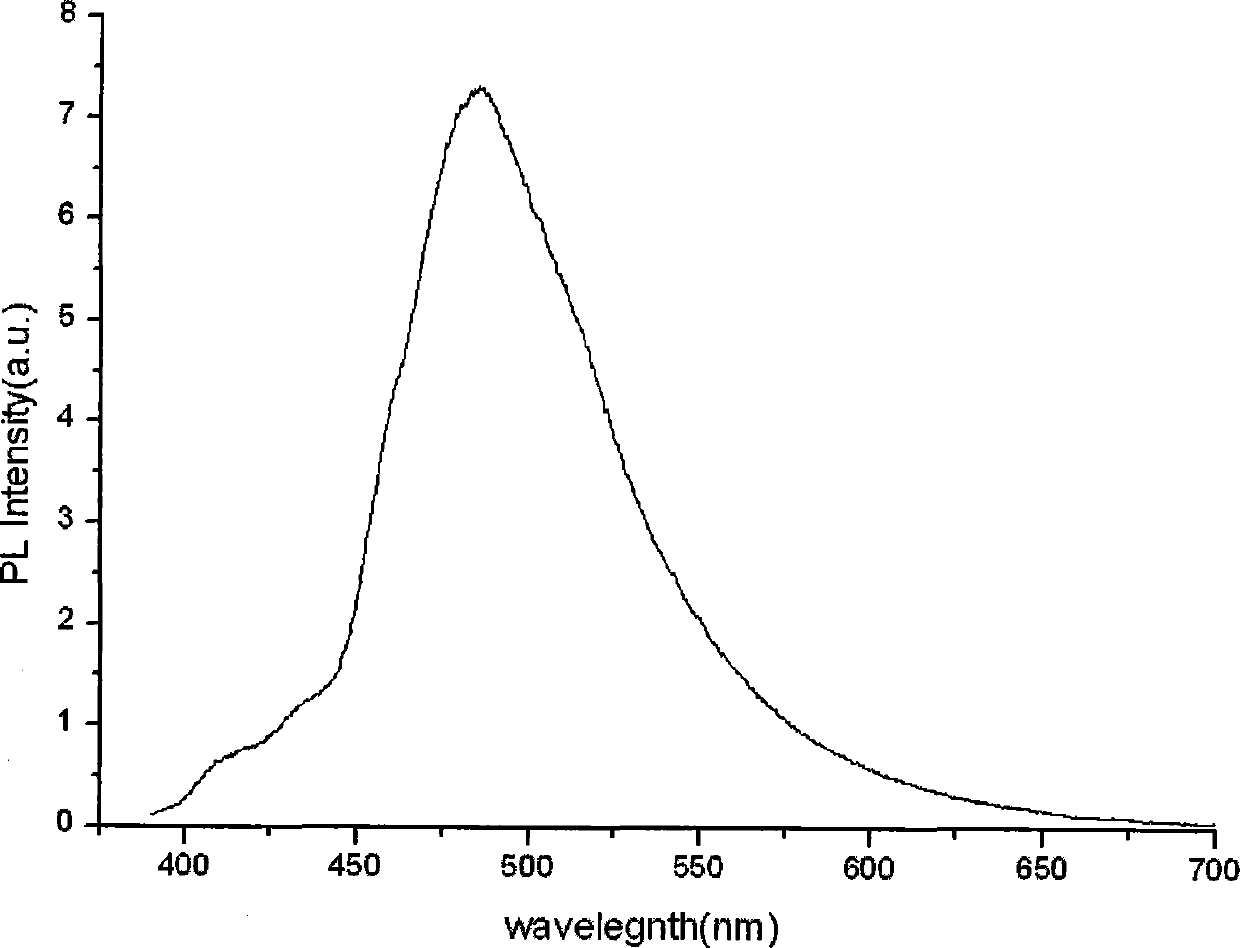
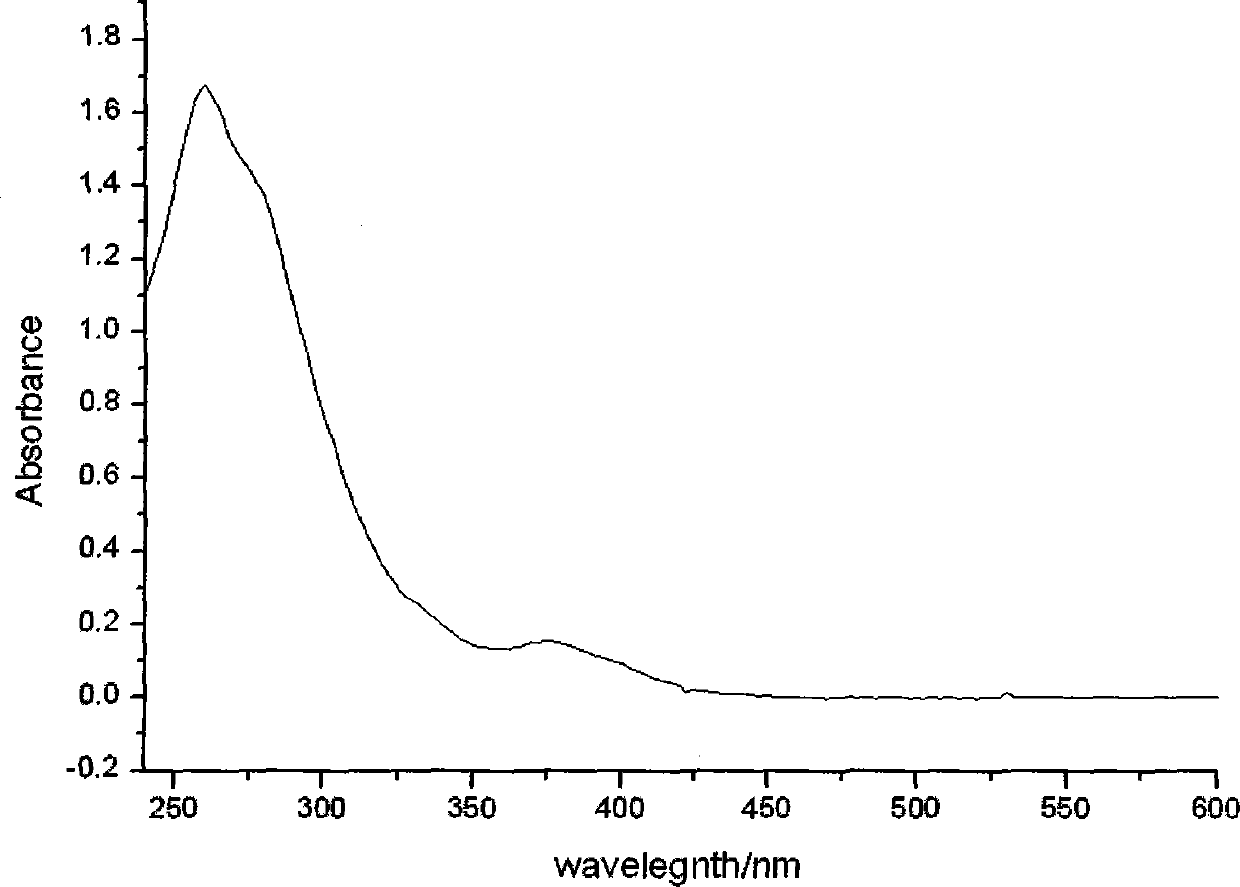
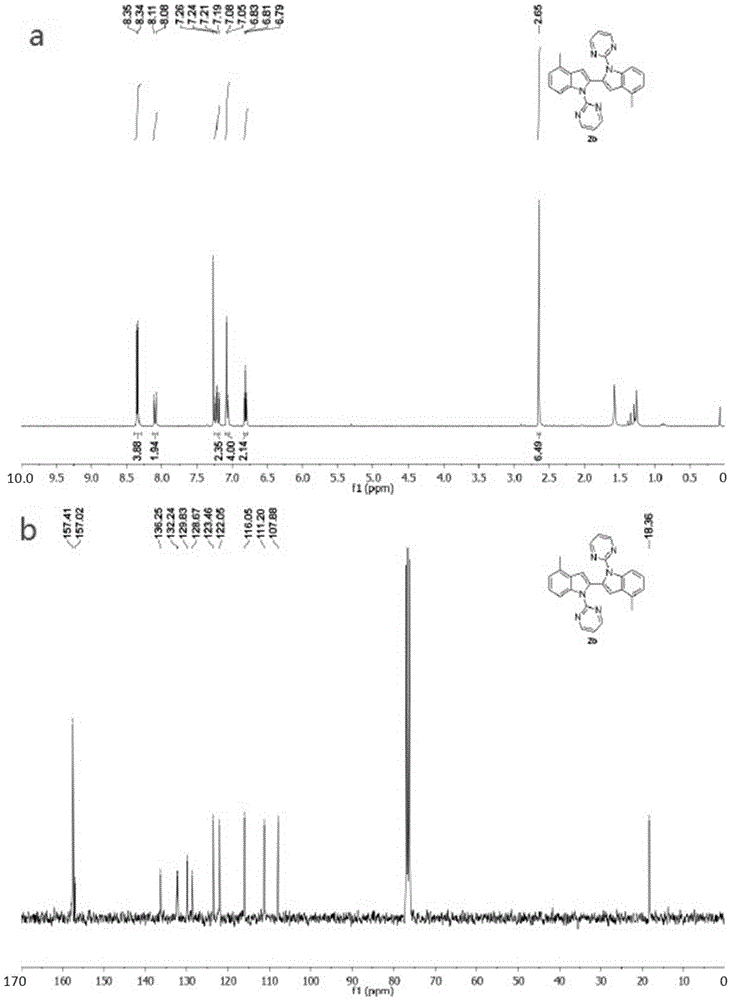
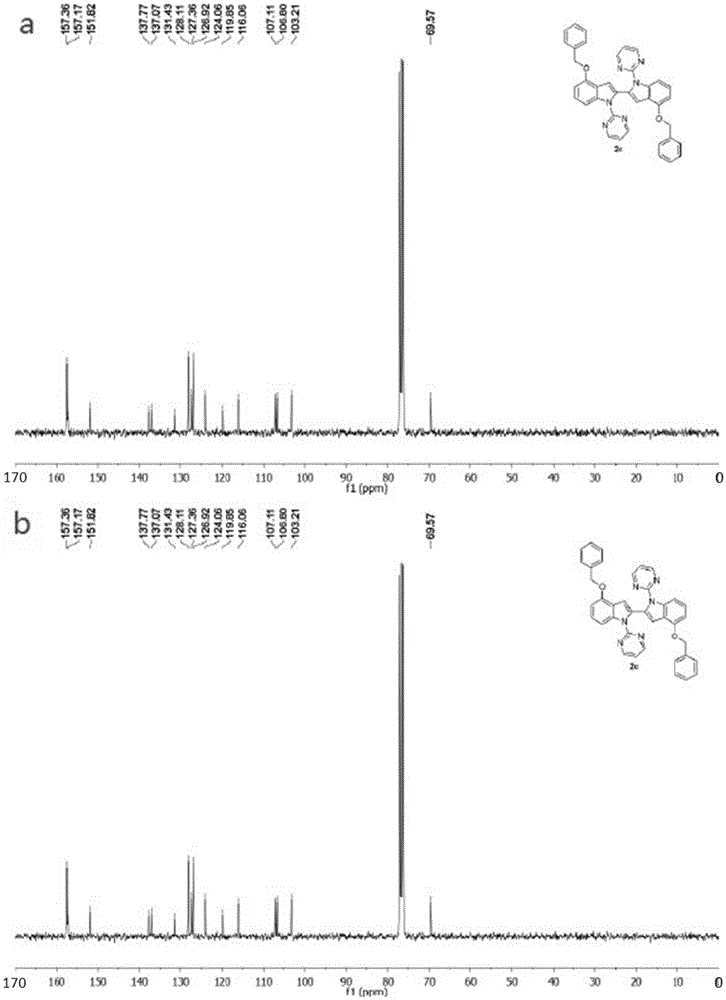
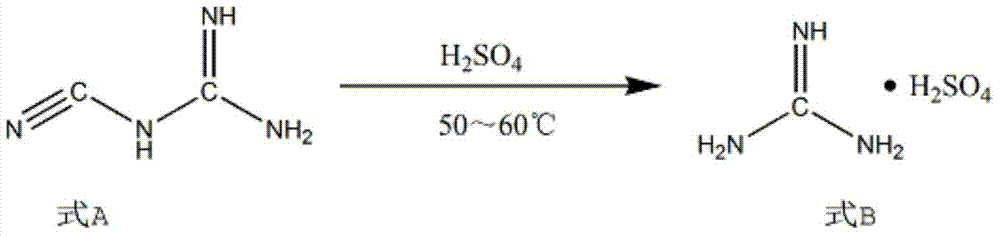
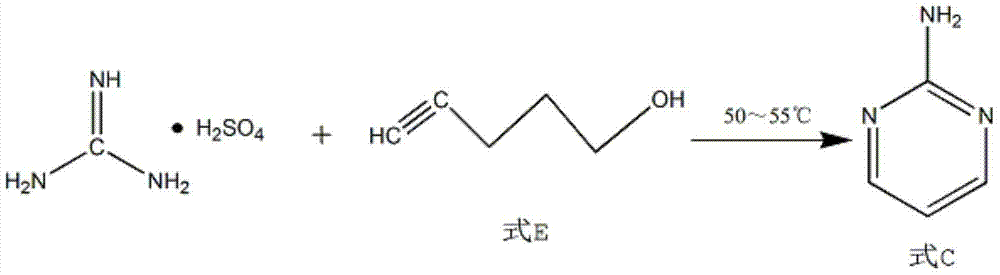
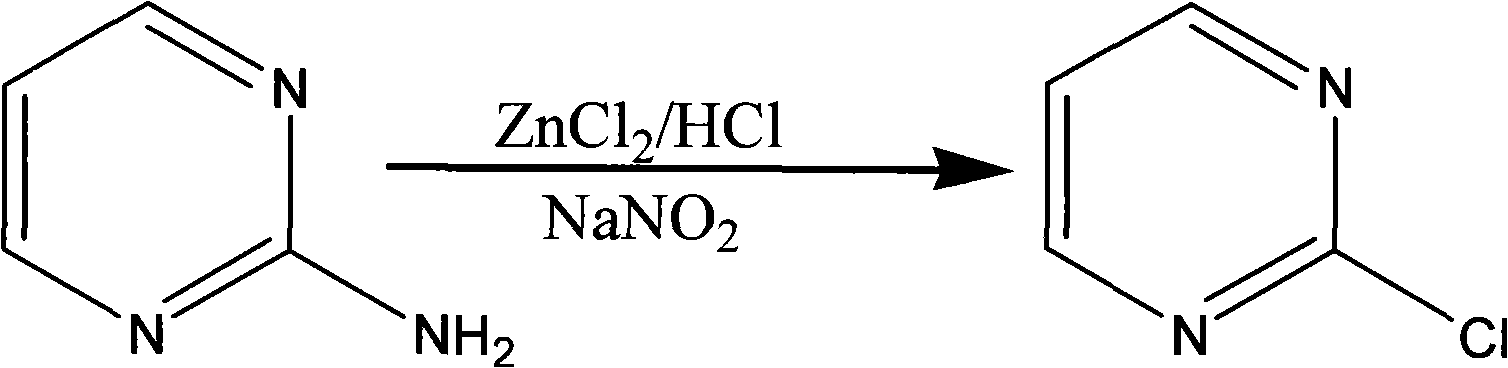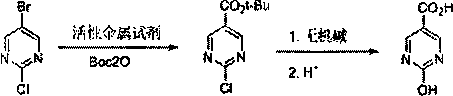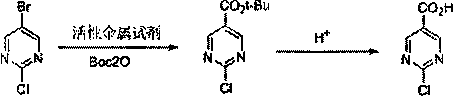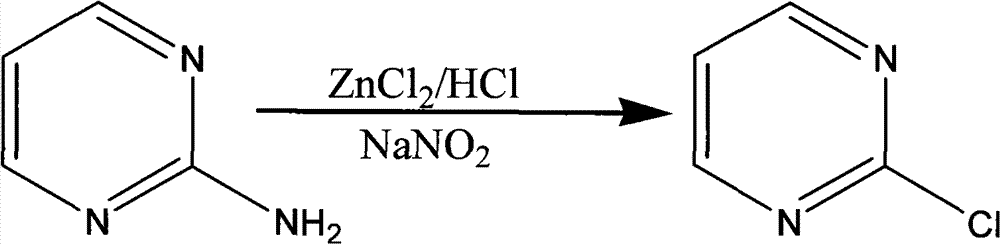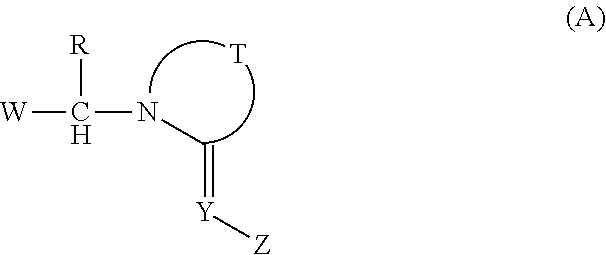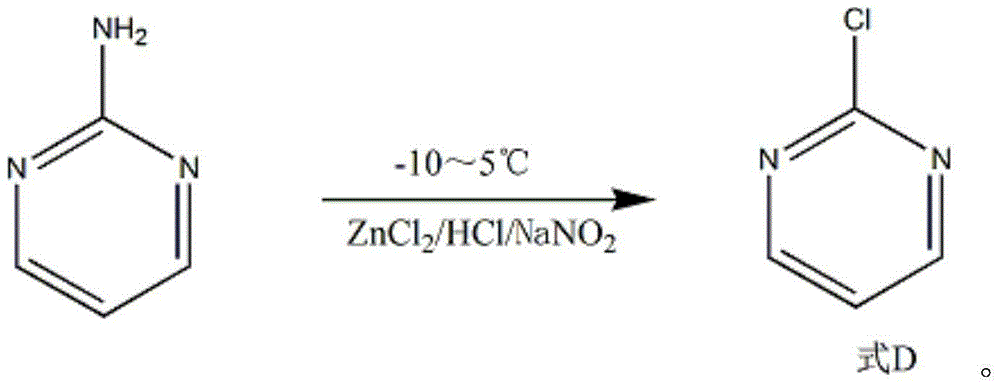Patents
Literature
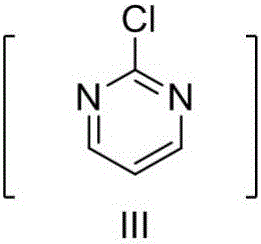
37 results about "2-chloropyrimidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
2-Chloropyrimidine undergoes cobalt-catalyzed cross-coupling reaction with aryl halides. Safety & Documentation. Safety Information. Symbol GHS07. Signal word Warning. Hazard statements H302-H319. Precautionary statements P301 + P312 + P330-P305 + P351 + P338. Personal Protective Equipment ...
Synthetic method of anti-tumor medicine
ActiveCN104817541AHigh yieldMild reaction conditionsCarbamic acid derivatives preparationOrganic compound preparationCarbamateMethyl-1H-indole
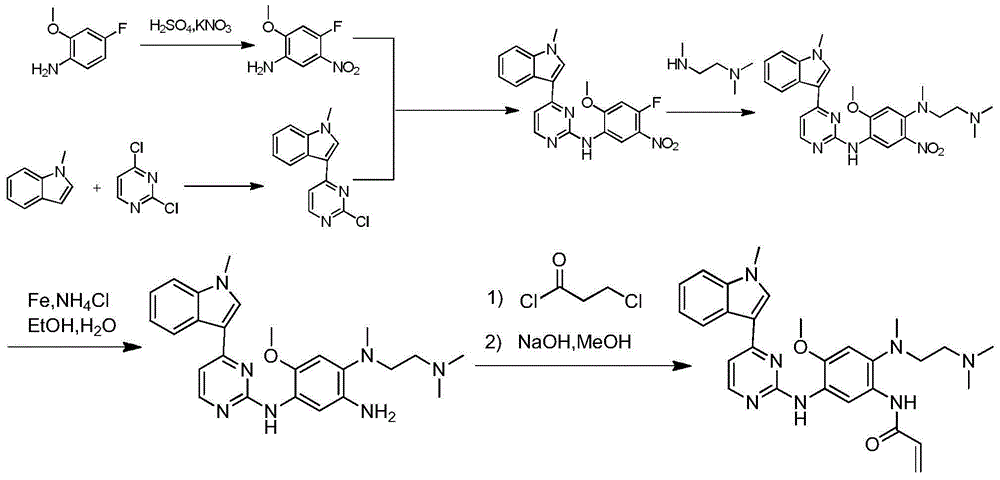
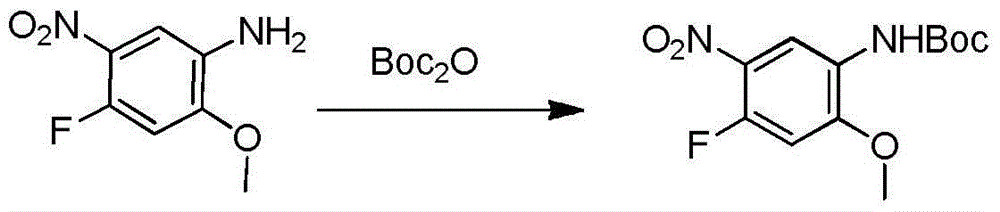
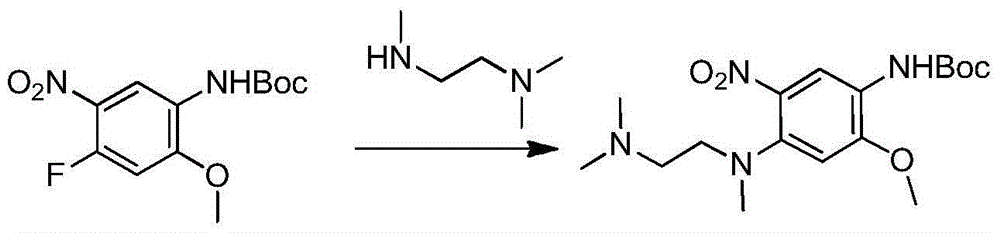
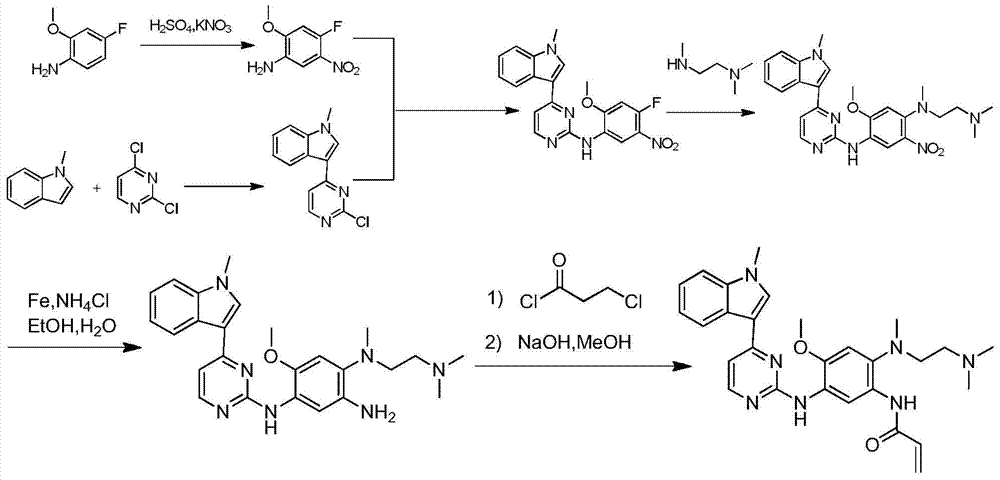
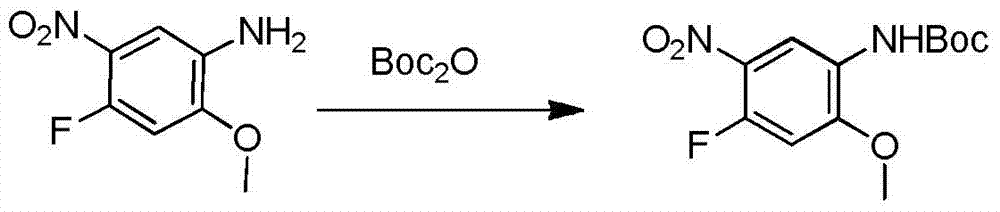
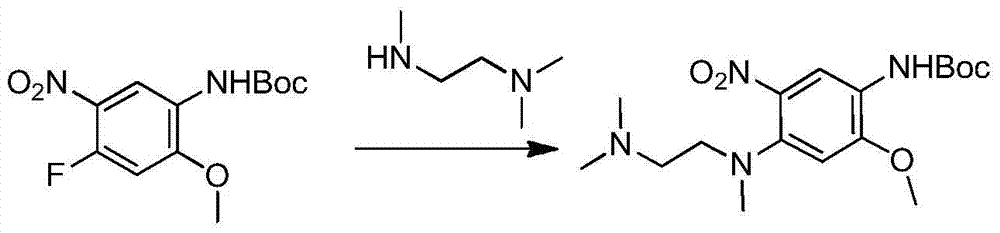
The invention relates to a synthetic method of an anti-tumor medicine, namely N-[2-[[2-(dimethylamino) ethyl] methyl amino]-4-methoxy-5-[[4-(1-methyl-1H-indole-3-yl)-2-pyrimidyl] amino] phenyl]-2-acrylamide (AZD9291) and a key intermediate of the anti-tumor medicine. The synthetic method comprises the following steps: performing Boc acid anhydride protection on 4-fluoro-2-methoxy-5-nitroaniline to obtain 4-fluoro-2-methoxy-5-nitroanilino tert-butyl formate, then reacting with N,N,N'-trimethylethylenediamine to obtain 4-(N,N,N'-trimethylethylenediamino)-2-methoxy-5-nitroanilino tert-butyl formate, then reducing to obtain 2-(N,N,N'-trimethylethylenediamino)-4-methoxy-5-tert-butyl carbamate phenylamine, then completely reacting with acryloyl chloride and directly removing a Boc protecting group to obtain 2-methoxy-4-N,N,N'-trimethylethylenediamino-5-acrylamido phenylamine, and finally reacting with 3-(2-chloropyrimidine-4-yl)-1-methylindole to obtain AZD9291. A process disclosed by the invention is simple in step, relatively high in yield, mild in reaction condition and easy for realization of industrial production.
Owner:苏州东南药业股份有限公司
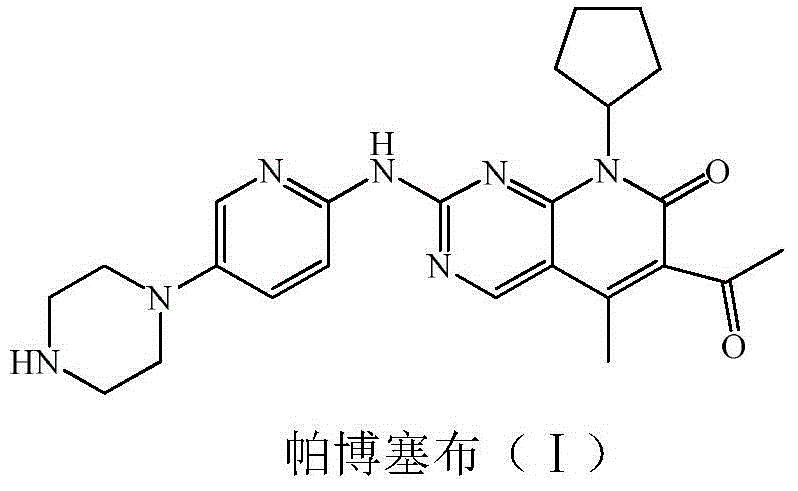
Low-cost preparation method for palbociclib
ActiveCN104610254ARaw materials are easy to getSimple processOrganic chemistryBulk chemical productionKetoneCarboxylic acid
The invention relates to a low-cost preparation method for palbociclib. The low-cost preparation method comprises the following steps: by taking 2,4-dichloro-5-cyanopyrimidine as an initial raw material, performing cyano Grignard and hydrolysis so as to obtain 5-acetyl-2,4-dichloropyrimidine, protecting carbonyl, performing amination, removing carbonyl, performing acylation reaction by using diketene and amino so as to obtain 5-acetyl-4-acetoacetamide-2-chloropyrimidine, performing intramolecular dehydration on the product so as to obtain 6-acetyl-8-cyclopentyl-5-methyl-2-chlorine-8H-pyridino-[2,3-d] pyrimidine-7-ketone, protecting 6 acetyl groups by using carbonyl, further realizing reaction with an aminopyridine derivative so as to obtain 4-{6-[(6-1,1 dialkoxyl) ethyl-8-cyclopentyl-5-methyl-7-keton-7,8 dihydropyridino-[2,3-d]pyrimidine-2-amino]-pyridine-3-yl}-piperazine-1-carboxylic acid tert-butyl ester, and finally performing acid hydrolysis and neutralization to obtain palbociclib free alkali. By adopting the low-cost preparation method, the raw materials are easy to obtain, the cost is low, and the environment can be protected.
Owner:XINFA PHARMA
Method for preparing 2-chloropyrimidine
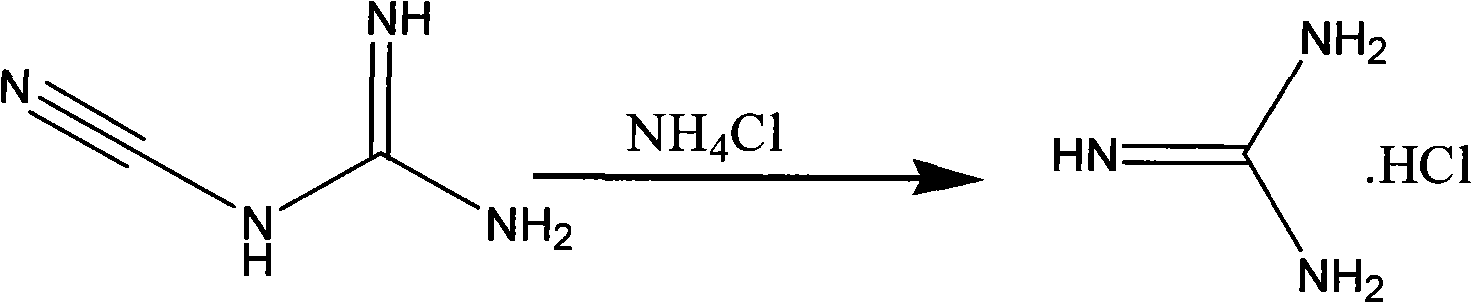
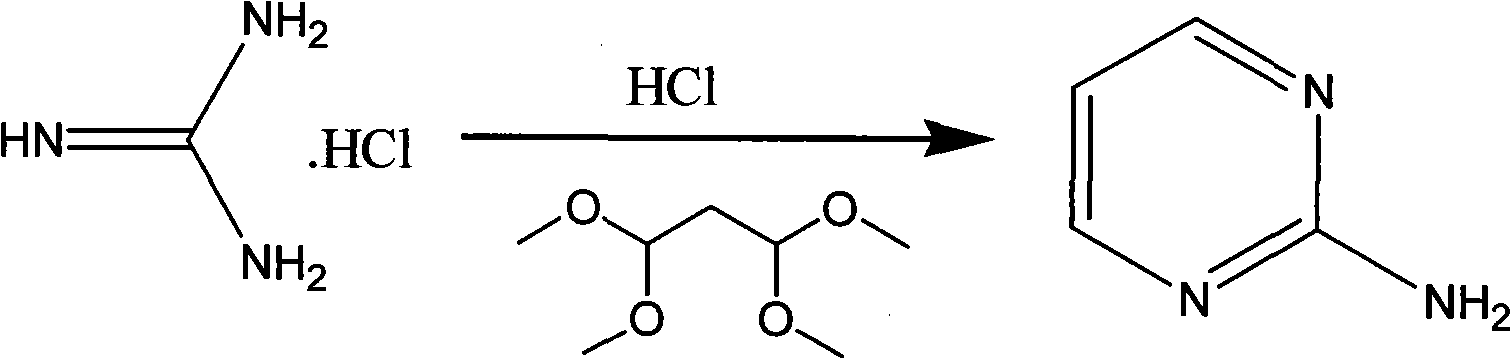
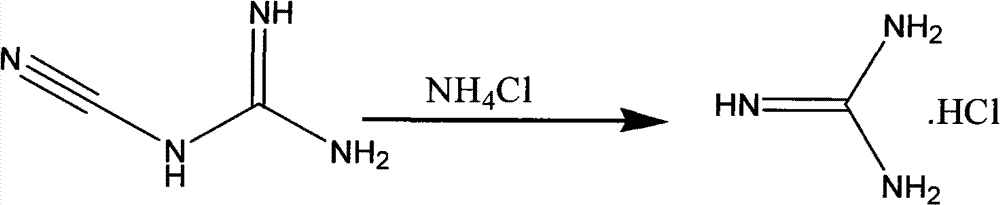
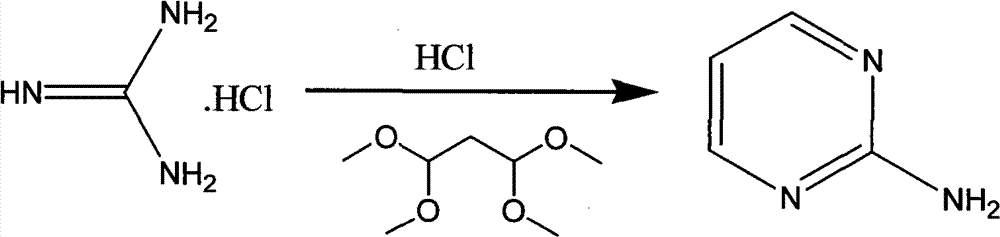
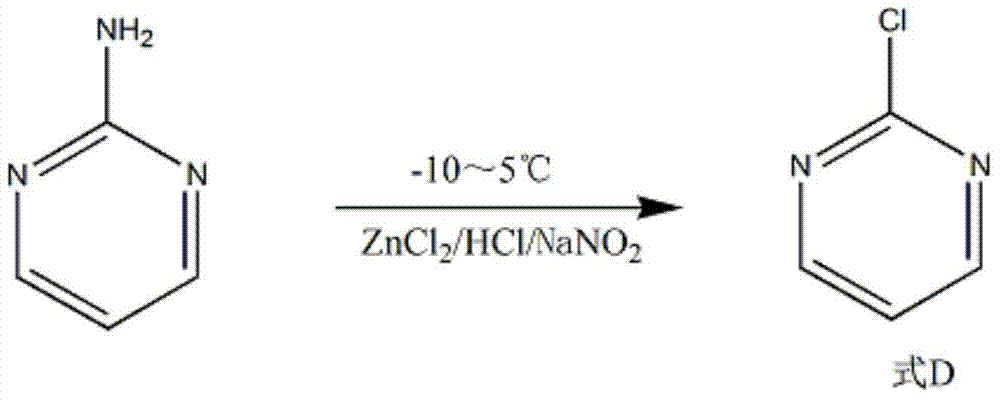
The invention discloses a method for preparing 2-chloropyrimidine, belonging to the technical field of methods for preparing chemical medicine intermediates. The method for preparing the 2-chloropyrimidine comprises the following steps of: (1) reacting dicyandiamide with ammonium chloride at high temperature, and obtaining guanidine hydrochloride; (2) reacting the guanidine hydrochloride with 1.1.3.3-tetramethoxypropane by refluxing under the condition of taking industrial hydrochloric acid as a solvent to obtain 2-amino pyrimidine; and (3) reacting the 2-amino pyrimidine with sodium nitrate at low temperature for producing the 2-chloropyrimidine by taking zinc chloride as a catalyst and taking the industrial hydrochloric acid as the solvent. The preparation method disclosed by the invention has the advantages of lower cost of raw materials, relatively simple technical process, mild reaction conditions, low operation difficulty, excellent maneuverability; convenience for post-treatment; and largely improved yield stable quality and applicability of large-scale industrial production of prepared products,.
Owner:LINHAI TIANYU PHARMA
Preparation method of piribedil
InactiveCN101735201AMild reaction conditionsLow costOrganic chemistryPurification methodsHigh pressure
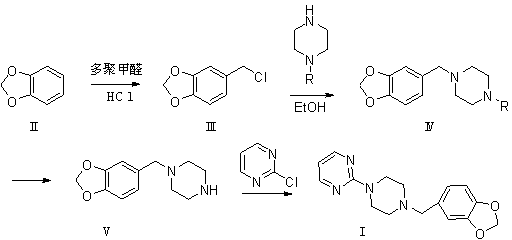
The invention relates to a preparation method of piribedil. The method is characterized in that piperidine is used as a starting raw material, and piperonyl chlorine is prepared through a Blanc reaction; in addition, 2-cloro pyridine is used as a starting raw material to carry out single alkylation on piperazine to obtain 1-(2-pyrimidyl)piperazine; and the prepared piperonyl chlorine and the 1-(2-pyrimidyl)piperazine are subjected to an N-alkylation reaction to obtain the piribedil. The invention has the advantages that the raw material piperidine has low price and is easy to obtain; the reaction condition is moderate, and reactions of all steps can be quickly carried out at lower temperature without high temperature, high pressure or special catalysts; the yield of products is higher; a purification method is simple; the purity is better (the purity content of HPLC is not lower than 99.8 percent), and the like, thus the method is a route which has simple operation, higher yield, lower cost and stable quality and is suitable for industrial production.
Owner:KANGYA OF NINGXIA PHARMA
Preparation method for selective kinases inhibitor Palbociclib
ActiveCN105153149AAvoid Hard to Buy Hard to BuyAvoid disadvantages such as hard to getOrganic chemistryN-methylacetamideTert-Butyloxycarbonyl protecting group
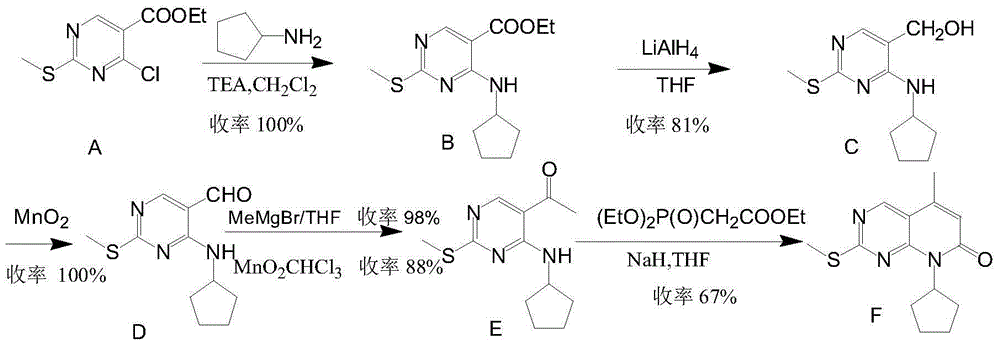
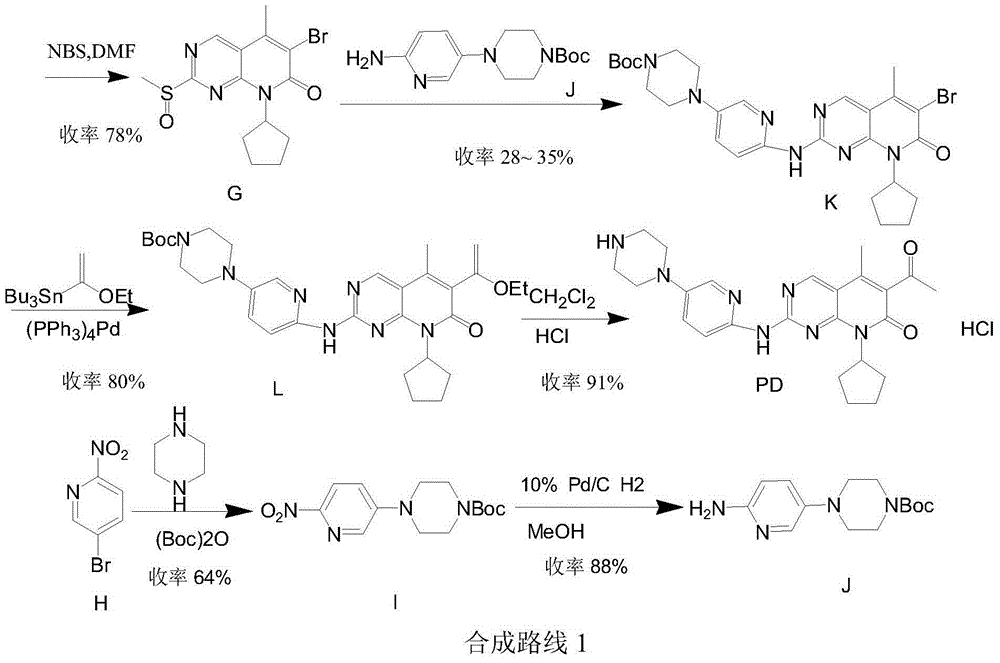
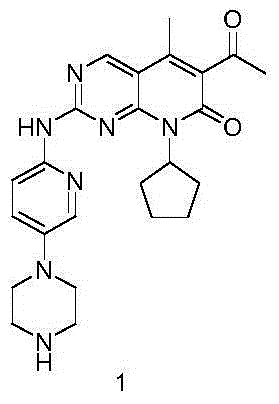
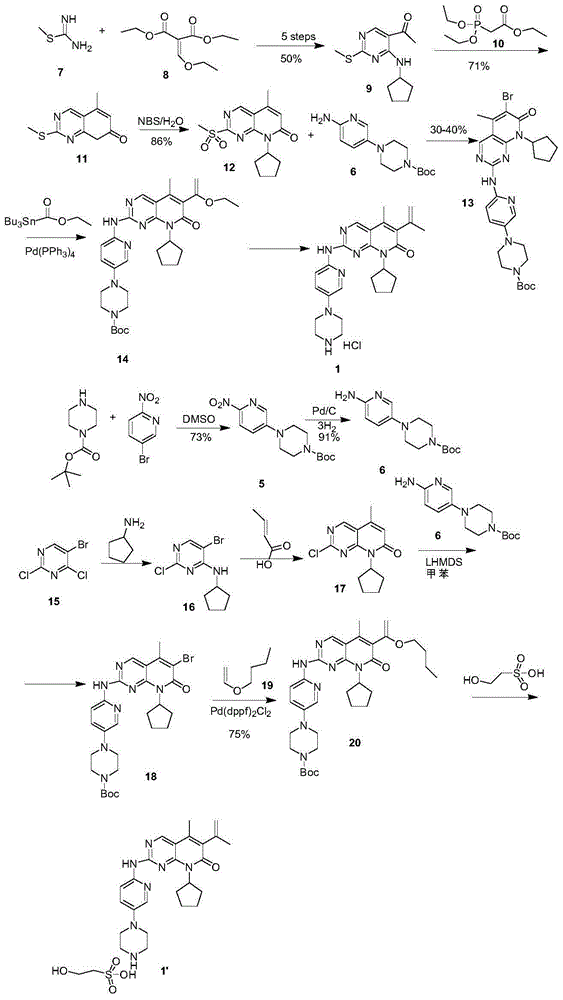
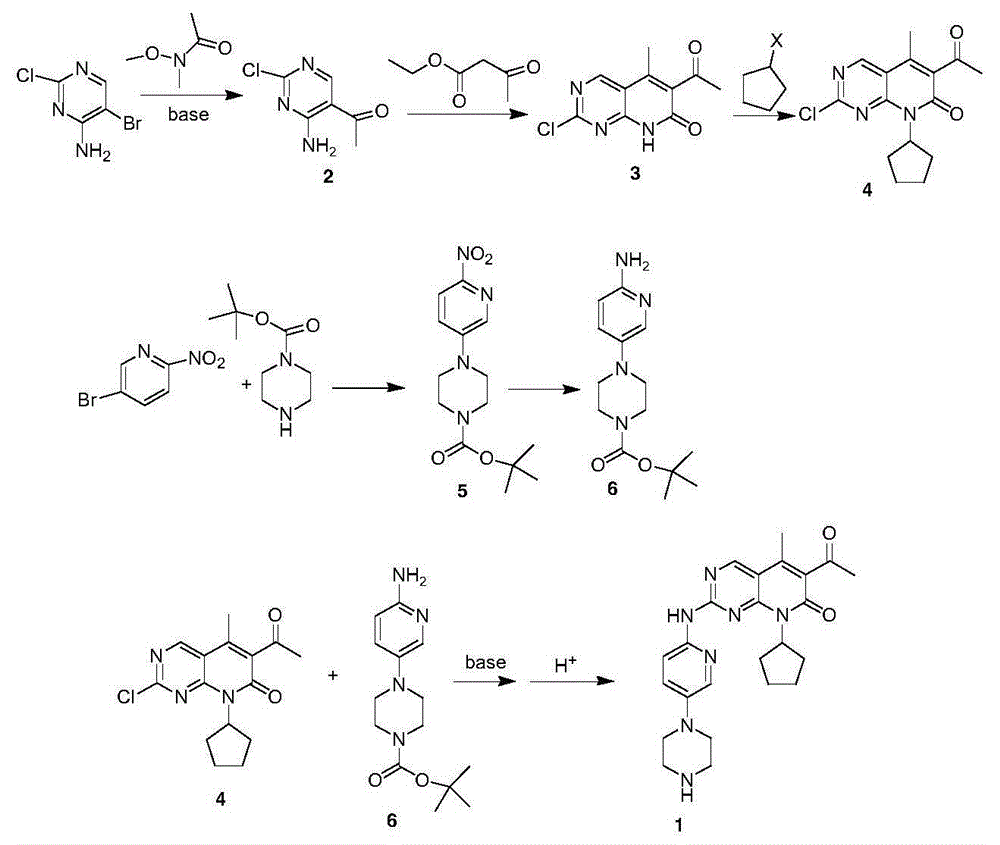
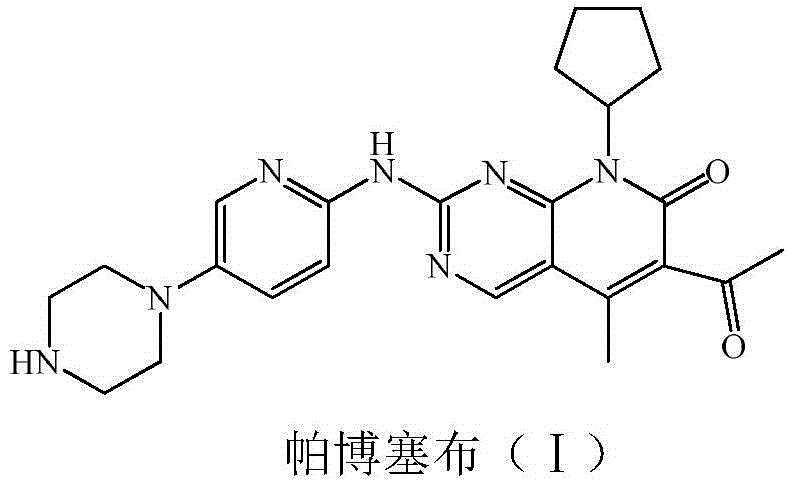
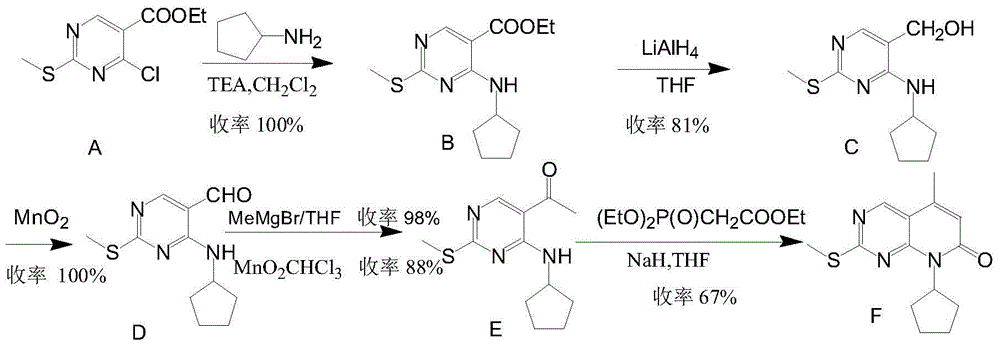
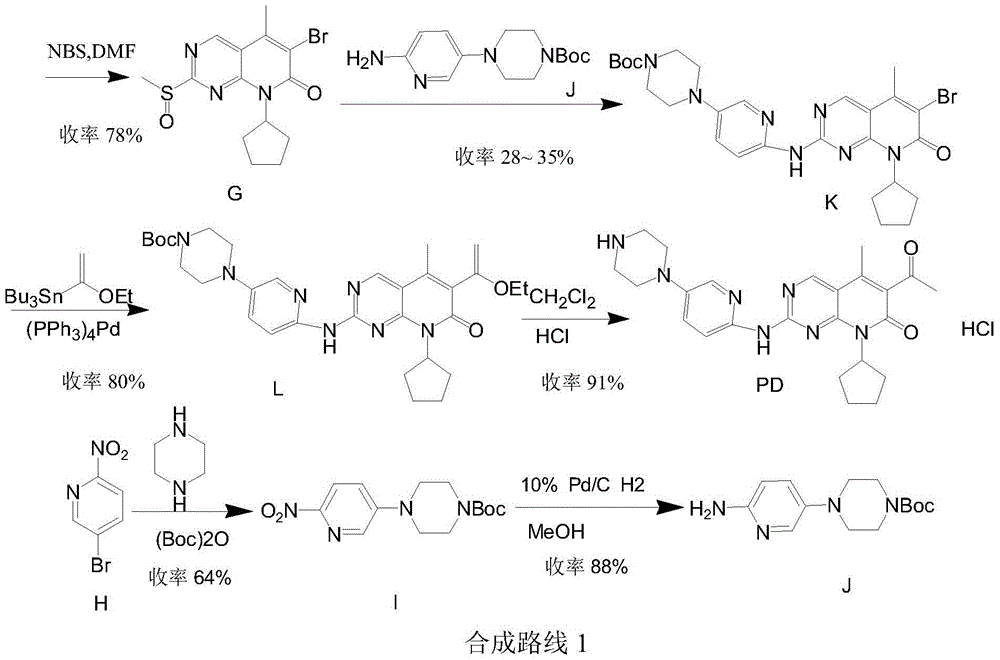
The invention provides a novel preparation method for a selective kinases inhibitor Palbociclib for cyclin-dependent kinases CDK4 and CDK6, and belongs to the technical field of medicament preparation. The preparation method comprises taking 4-amino-5-bromo-2-chloropyrimidine as an initial raw material, performing acetylation with N-methoxy-N-methylacetamide to obtain an intermediate 2, performing Friedlaender reaction on the intermediate and a raw material ethyl acetoacetate to obtain an intermediate 3, and performing amino substitution on the intermediate 3 and a halogenated cyclopentane to obtain an intermediate 4; performing substitution reaction on a raw material 5-bromo-2-nitropyridine and a raw material tert-butyl 1-piperazinecarboxylate to obtain an intermediate 5, and reducing the intermediate 5 to obtain an intermediate 6; performing substitution reaction on the intermediate 4 and the intermediate 6, and performing deprotection to obtain the target product 6-acetyl-8-cyclopentyl-5-methyl-2-[[5-(piperazin-1-yl)pyridin-2-yl]amino]-8H-pyrido[2,3-d]pyrimidin-7-one with the yield of 45% or more. A brand-new route is employed, the reaction raw materials are cheap and easy to obtain, reaction conditions are mild, noble metal catalysts are avoided, and the preparation method is suitable for industrialized production.
Owner:JIANGSU ZHONGBANG PHARMA
Method for preparing piribedil in high-purity high-yield manner
The invention discloses a new method for preparing piribedil in a high-purity high-yield manner. The method is characterized by comprising the following steps of: utilizing piperidine as a raw material, and preparing the piribedil through four-step reaction, namely a blanc chloromethylation, an N-single protection piperazine ammoniation, deprotection and 2-cloro pyridine condensation. Compared with the traditional method, the method provided by the invention has the advantages that the N-single protection piperazine replaces piperazine, the piperazine dosage is reduced, a side reaction is alleviated, the impurities of a reaction process are reduced, the operation is simple, the condition is mild and is easy to control, the aftertreatment is convenient, according to the method, the environmental protection is realized, the total recovery is high, and the method is novel and used for industrially compounding piribedil.
Owner:安徽安腾药业有限责任公司
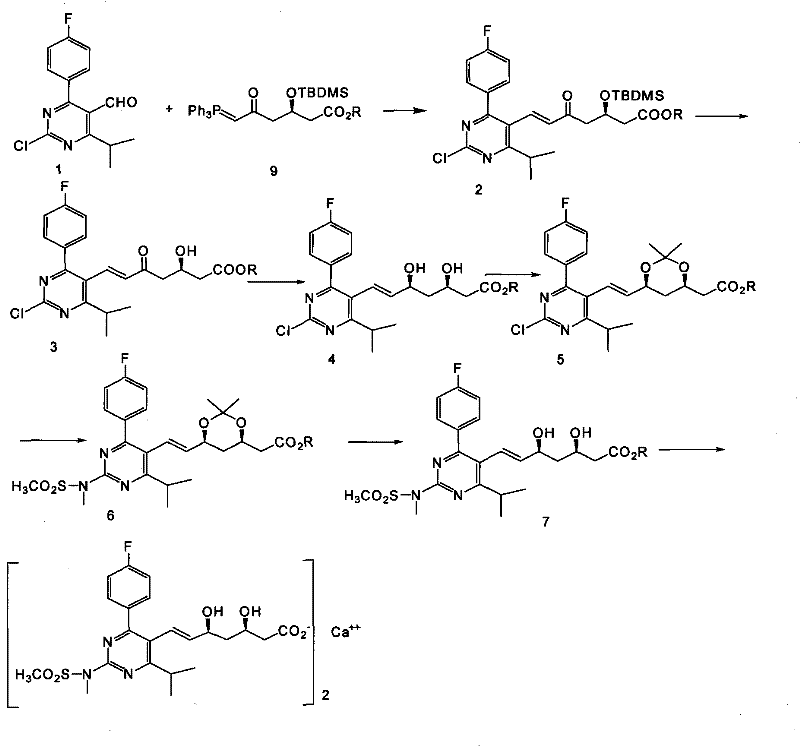
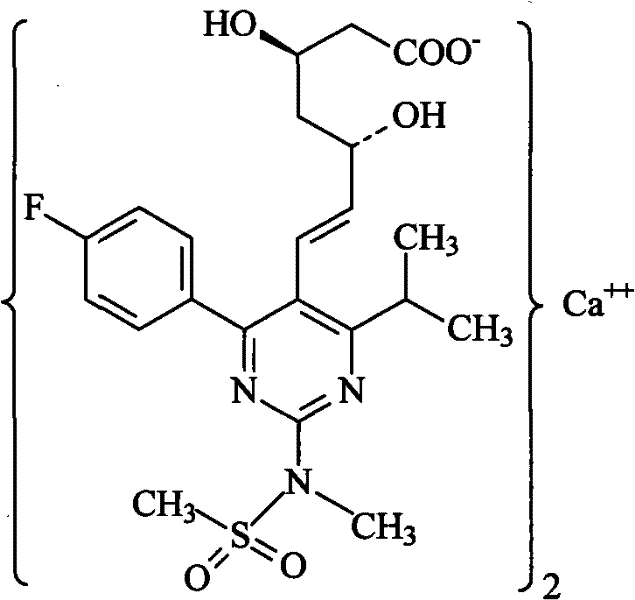
Method for preparing rosuvastatin calcium
ActiveCN102219749ALow priceHigh yieldOrganic chemistryBulk chemical productionRosuvastatin CalciumProtecting group
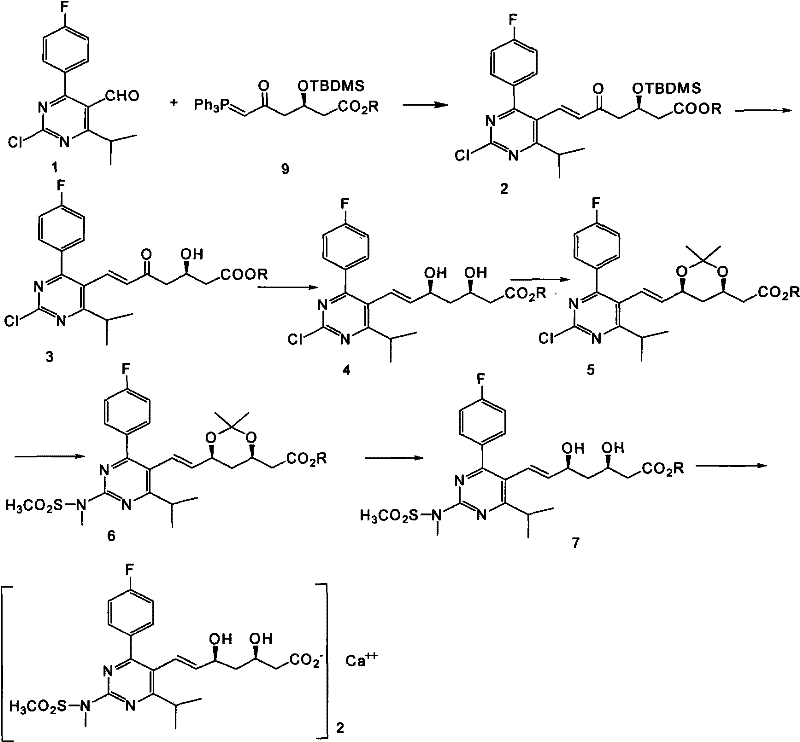
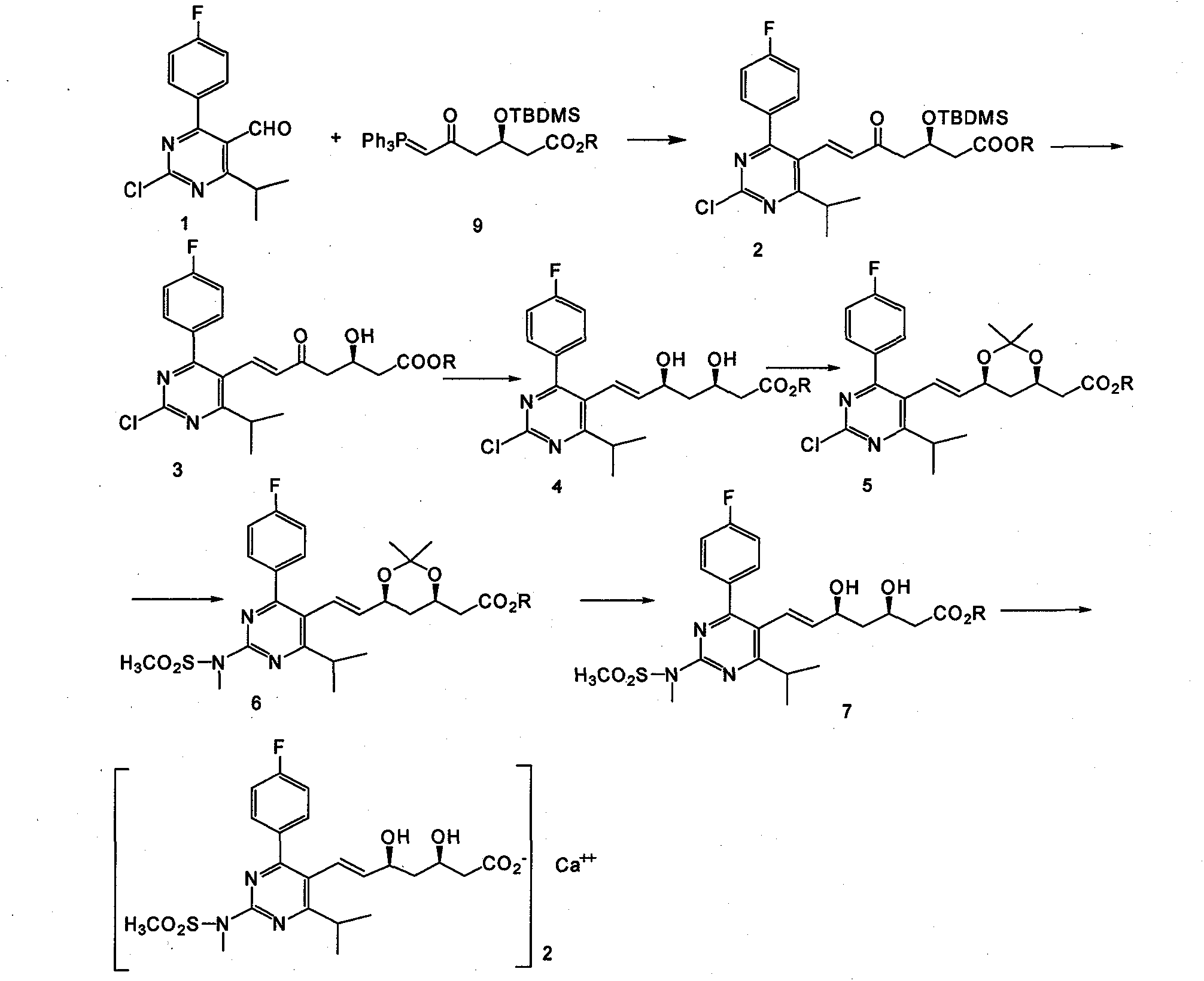
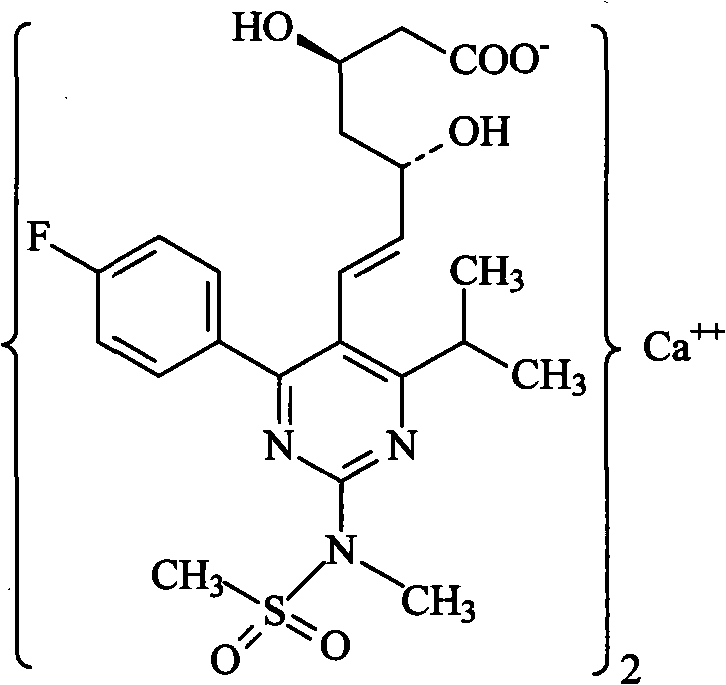
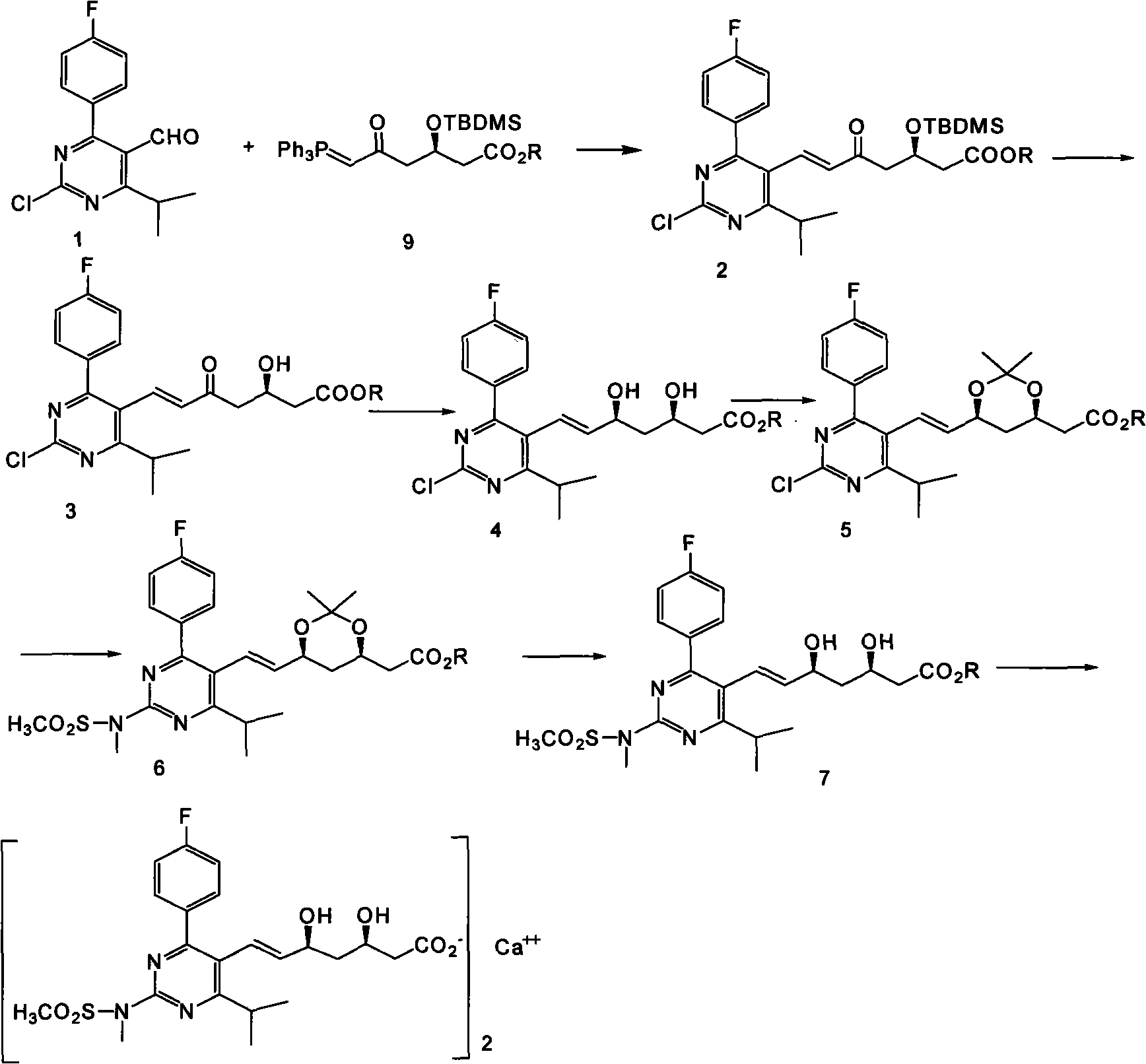
The invention provides a preparation method of rosuvastatin calcium. The method comprises the following steps: carrying out condensation reaction on 4-(4-fluorophenyl)-6-isopropyl-2 chloropyrimidine-5-formaldehyde and (3R)-tertiary butyl dimethylsiloxo-5-oxo-6-triphenyl phosphaalkene hexanoate; and then removing a silylether protecting group of hydroxyl, carrying out stereoselectivity reduction, reacting with 2,2-dimethoxy propane, condensing with N-methyl methane sulfonamide, removing propylidene protection, hydrolyzing an ester group, salifying and carrying out other steps, so as to obtain the rosuvastatin calcium. According to the preparation method, the yield is stable, and the price of reagents is cheap; and the method is easy to operate and is beneficial to industrial production.
Owner:ZHEJIANG JINGXIN PHARMA
Preparation method of pyridylaminopyrimidine derivative and intermediate thereof
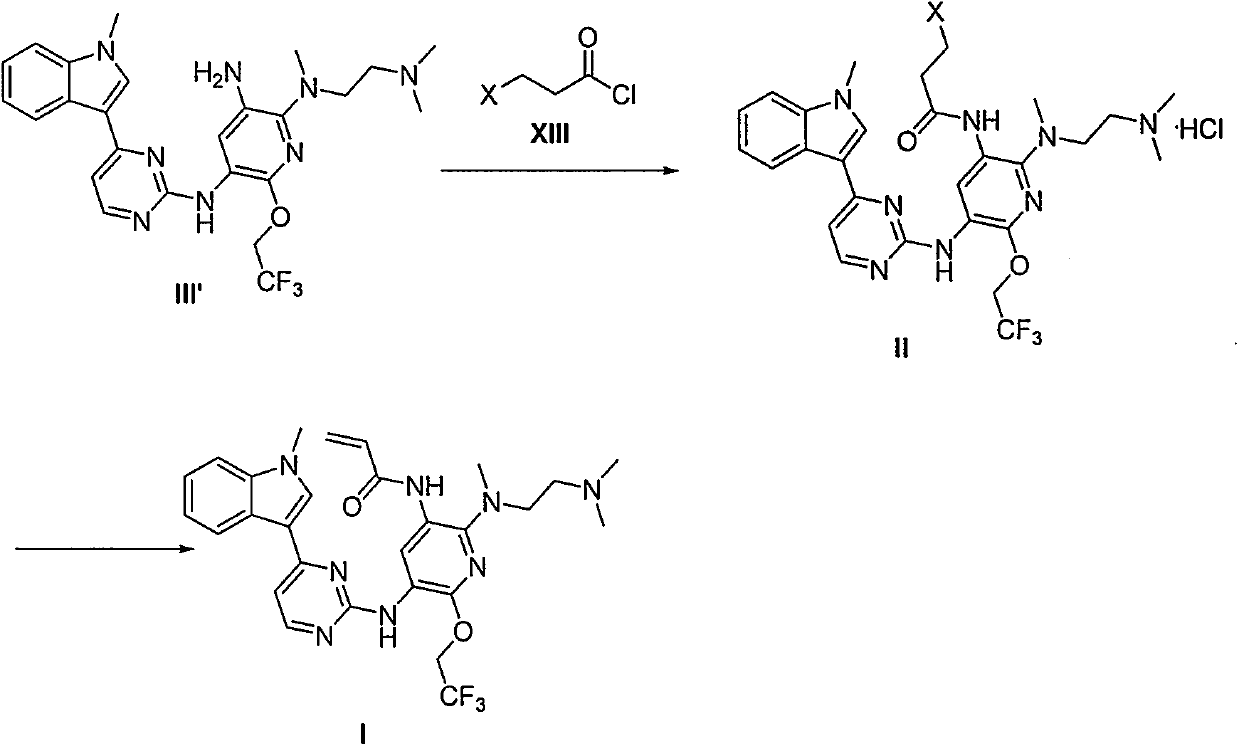
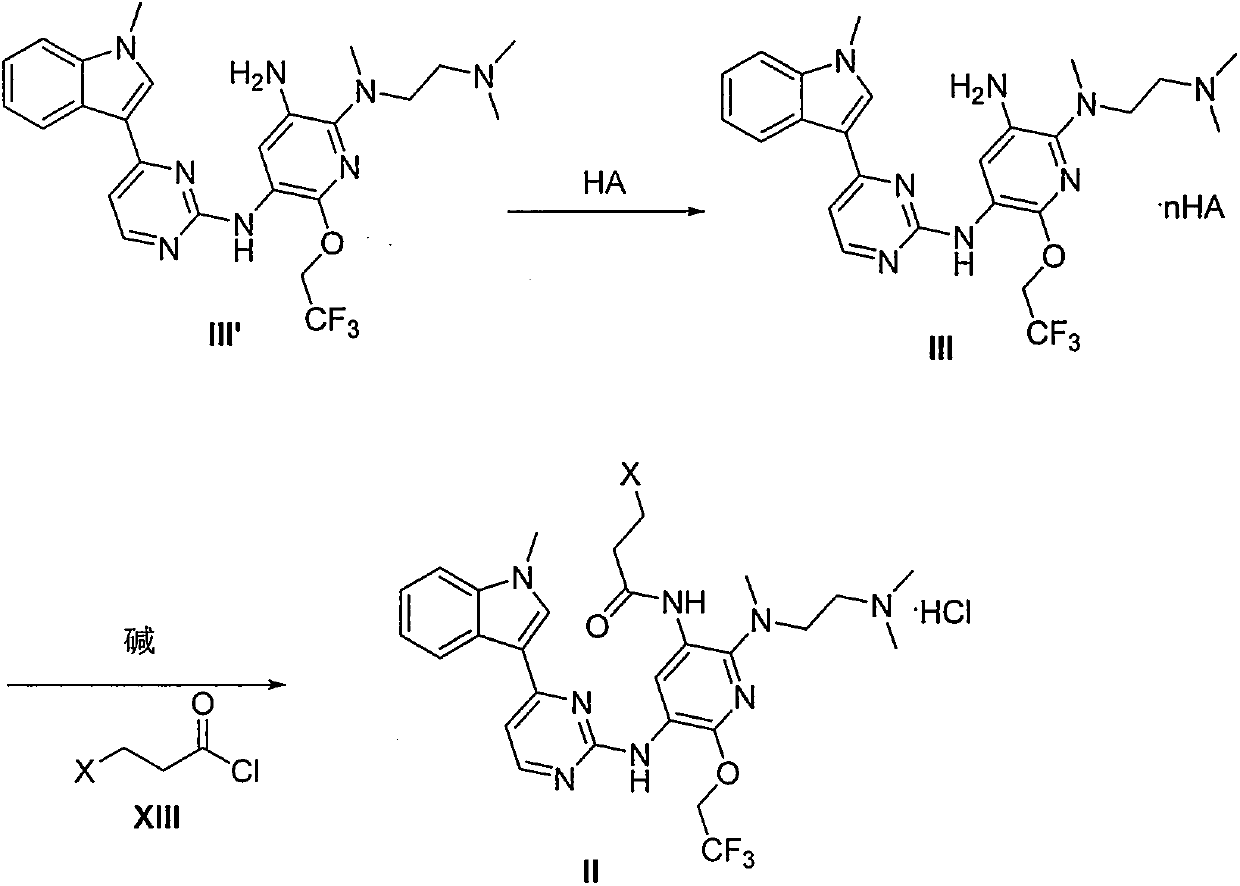
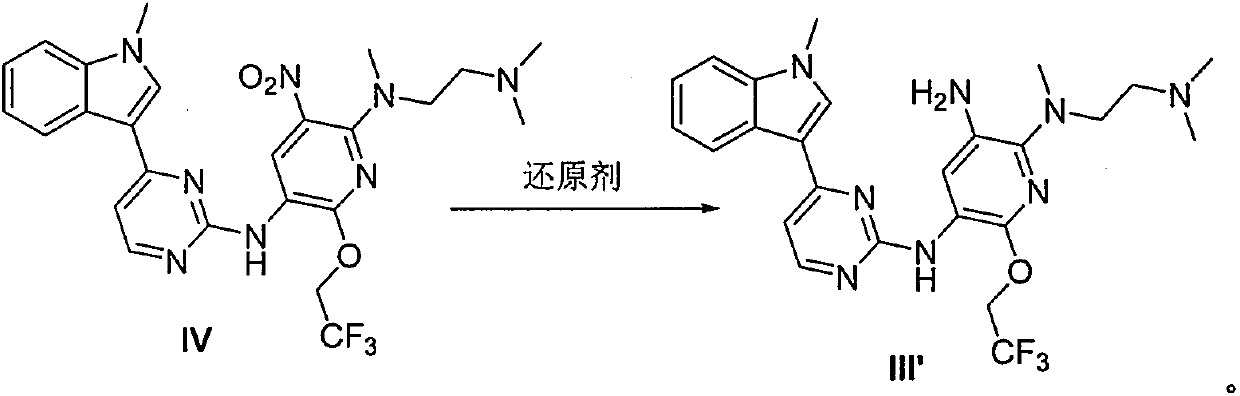
The invention provides a preparation method of a compound 2-[2-(dimethylaminoethyl) methylamino]-3-acrylamido-5-[4-(1-methyl-1H-indole-3-yl) pyrimidine-2-amino]-6-(2, 2, 2-trifluoroethoxy) pyridine asshown in a formula I, a used intermediate and a preparation method of a related intermediate. According to the method, 3-(2-chloropyrimidine-4-yl)-1-methyl-1H-indole and a compound shown as a formulaVII are subjected to a condensation reaction, a substitution reaction, a reduction reaction, an acylation reaction and an elimination reaction, and a compound shown as a formula I is obtained. The preparation method disclosed by the invention is environment-friendly, low in cost, mild in condition, simple to operate, high in yield, high in final product purity and suitable for industrial production.
Owner:SHANGHAI ALLIST PHARM CO LTD +1
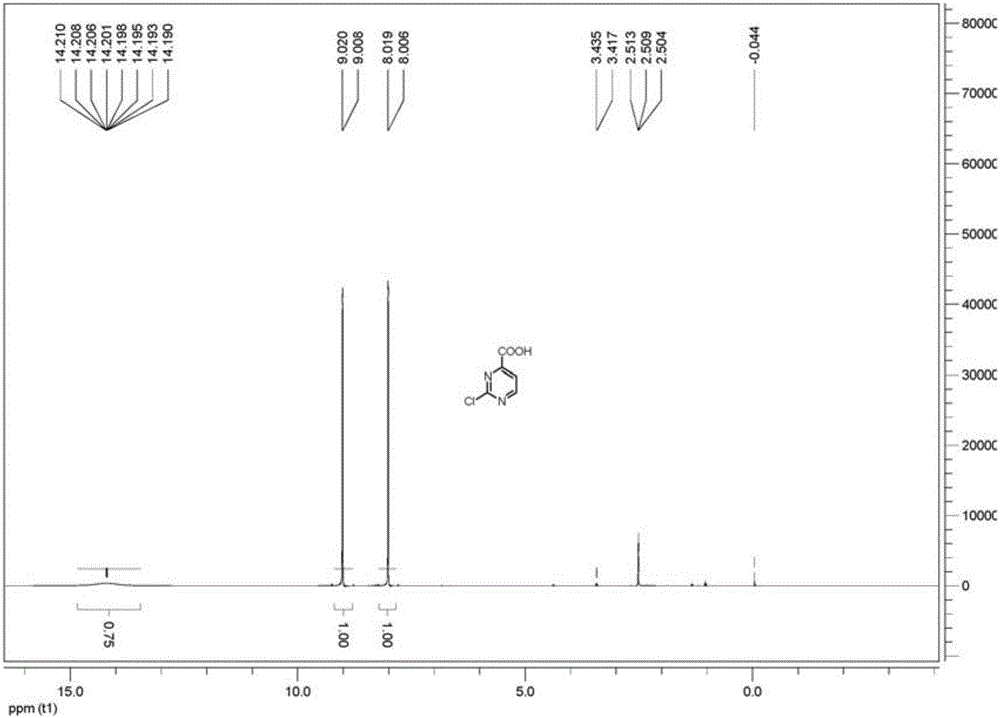
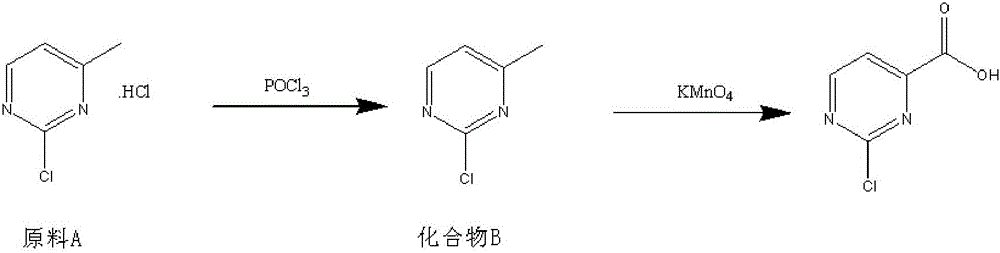
Method for preparing 2-chloropyrimidine-4 formic acid
The invention relates to a method for preparing 2-chloropyrimidine-4 formic acid. The method comprises the following steps: performing debydrochlorination reaction on 2-chlorine-4-methyl pyridine hydrochloride and phosphorus oxychloride and separating, thereby acquiring 2-chlorine-4-methyl pyridine; and performing methyl oxidizing reaction on 2-chlorine-4-methyl pyridine and methyl oxidant and separating, thereby acquiring 2-chloropyrimidine-4 formic acid. According to the process route provided by the invention, the yield of the target product is high, the process route can be amplified, the raw materials can be easily acquired and have low cost, and industrial production can be carried out.
Owner:安徽沛成医药科技有限公司
Method of preparing 2-chloropyrimidine
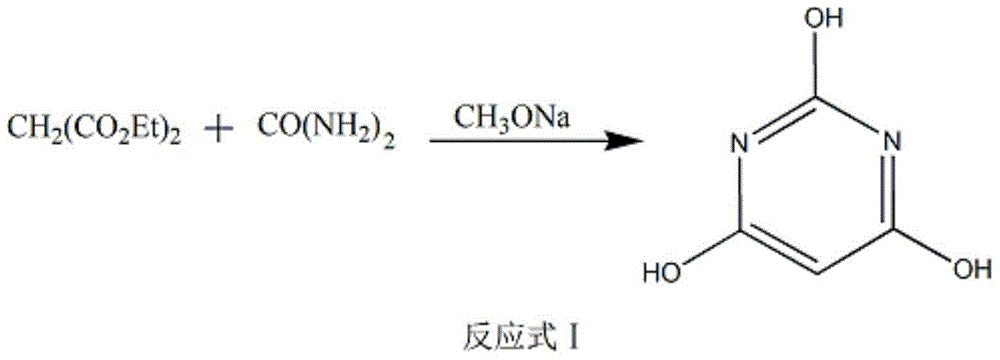
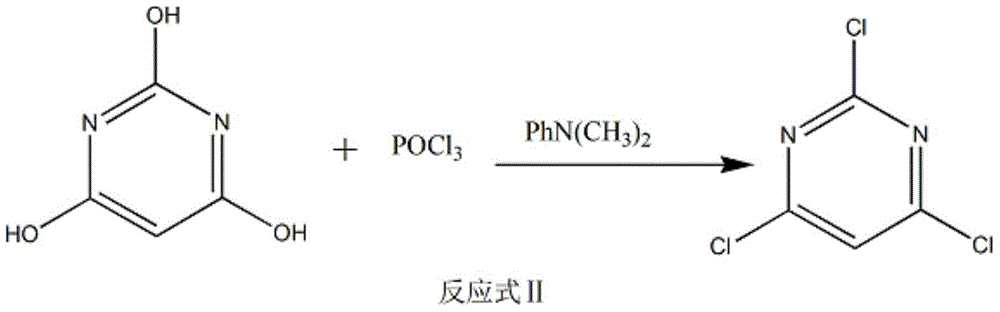
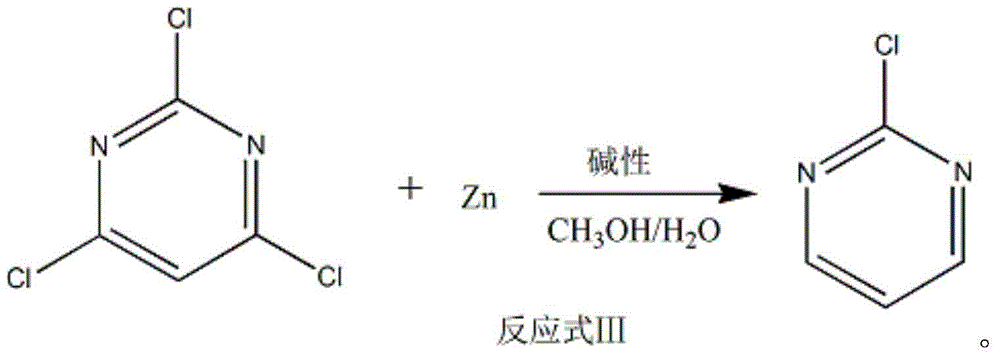
The invention discloses a method of preparing 2-chloropyrimidine. The method comprises the following steps: firstly carrying out reaction on malonic acid bis-ethylacetate and urea with sodium methoxide as a catalyst to obtain barbituric acid, then carrying out reaction on the barbituric acid and phosphorus oxychloride with N, N-dimethylaniline as a catalyst to obtain 2, 4, 6-trichloropyrimidine, and finally carrying out redox reaction on the 2, 4, 6-trichloropyrimidine and zinc powder in a solvent of methanol and water under the alkaline condition to obtain the 2-chloropyrimidine, wherein the molar ratio of the 2, 4, 6-trichloropyrimidine to the zinc powder is 1:(1-1.2). According to the method disclosed by the invention, by virtue of the 2, 4, 6-trichloropyrimidine and the zinc powder with the molar ratio to be 1:(1-1.2), only chlorine on 4 and 6 bits on a pyrimidine ring of the 2, 4, 6-trichloropyrimidine can be reduced, so that the side effect of generating dichloride or pyrimidine is reduced; and by virtue of the reaction route, the 2-chloropyrimidine with high purity and yield is obtained.
Owner:TAICANG YUNTONG BIOCHEM ENG
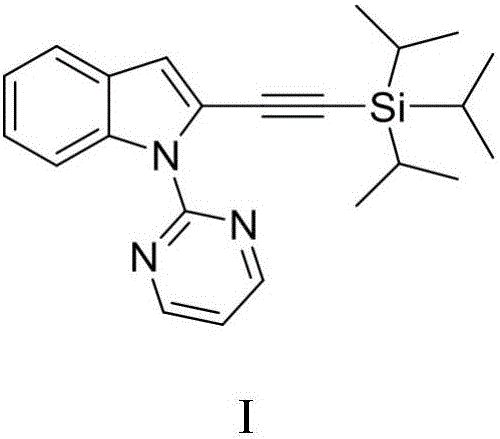
Method for synthesizing 2-triisopropyl silicon substrate acetylene indoles compound
InactiveCN106279236AImprove economyAvoid preactivationGroup 4/14 element organic compoundsPalladium catalystSolvent
The invention discloses a method for synthesizing a 2-triisopropyl silicon substrate acetylene indoles compound. The method comprises the steps of firstly adopting the indoles compound as a raw material, and under an alkaline condition of sodium hydride, generating a 1-(pyrimidyl-2-base)-1H-indoles compound through the reaction of indole or substituted indole and 2-chloropyrimidine; introducing a pyridine guiding group into the indoles compound; then carrying out alkynylation reaction on the 1-(pyrimidyl-2-base)-1H-indoles compound under the participation of bispalladium dichloride, cesium carbonate, silver hexafluoroantimonate, aluminium oxide, triisopropyl silicon substrate acetylene bromine and a 1,4-dioxane solvent; finally synthesizing the 2-triisopropyl silicon substrate acetylene indoles compound. The method applies a direct C-H activation reaction method, so that a pre-functionalization process is eliminated, reaction steps are reduced, and the reaction economical efficiency is improved.
Owner:NANJING UNIV OF SCI & TECH
Method for synthesizing 2-chloro/hydroxypyrimidine-5-carboxylic acid
ActiveCN109467536AGood operation reproducibilityHigh yieldProductsOrganic chemistryMetalloleRoom temperature
The invention discloses a method for synthesizing 2-chloro / hydroxypyrimidine-5-carboxylic acid, and belongs to the field of synthesis of pharmaceutical intermediates. The invention comprises the following steps: reacting 2-chloro-5-bromopyrimidine with Boc2O under action of an active metallic reagent; then, adding an aqueous acid solution or an aqueous alkali solution to reflux at room temperature; and treating to obtain 2-chloropyrimidine-5-carboxylic acid and 2-hydroxypyrimidine-5-carboxylic acid, respectively. The method avoids a problem of low yield during scale-up by a method of feeding CO2, has good batch-to-batch operation reproducibility, and provides a new way for production scale-up.
Owner:常州琦诺生物科技有限公司
Synthesis method of 2-chloropyrimidine
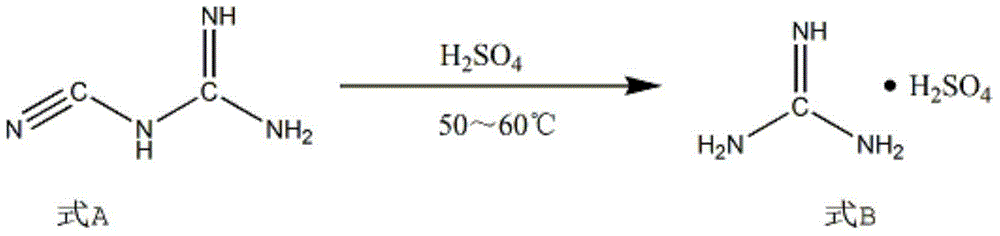
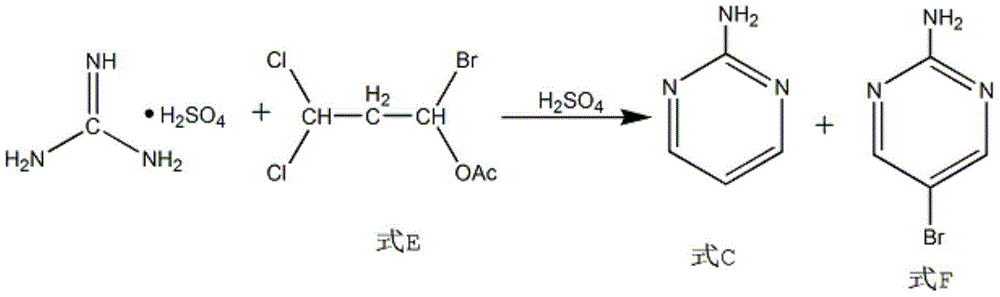
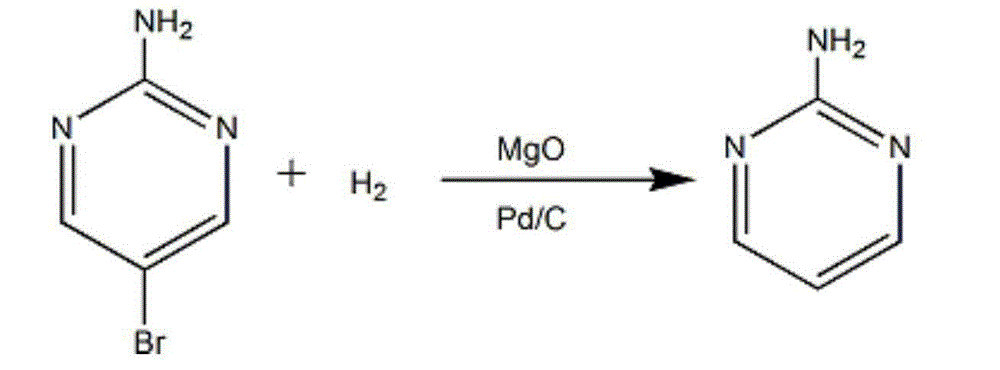
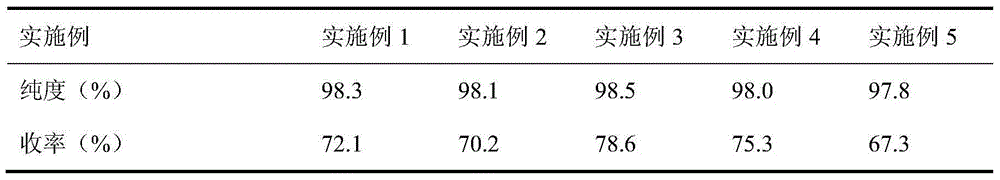
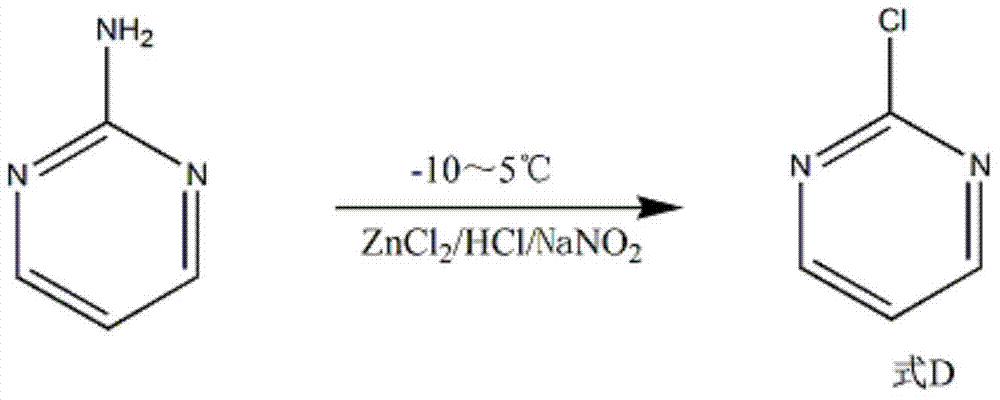
The invention discloses a synthesis method of 2-chloropyrimidine. The synthesis method comprises the steps of dissolving dicyandiamide in sulfuric acid at 2-6 DEG C, heating at 50-60 DEG C for hydrolysis reaction for 4-6h to form guanidine sulfate, allowing guanidine sulfate to carry out cyclization reaction with 1-bromine-1-acetyl-3,3-propylene dichloride (Formula E) for 5-8h at 50-60 DEG C in the presence of sulfuric acid at a mass concentration of 94-96wt% to form 2-aminopyrimidine and 2-amino-5-bromopyrimidine, hydrogenizing and reducing 2-aminopyrimidine and 2-amino-5-bromopyrimidine to 2-aminopyrimidine at 55-65 DEG C under the catalysis of a palladium-carbon catalyst and magnesium oxide at the same time, and reducing 2-aminopyrimidine to the product at subzero10-5 DEG C by taking NaNO2, HCl and ZnCl2 as catalysts. Through the preparation route, higher-purity and upper-yield 2-chloropyrimidine is obtained.
Owner:TAICANG YUNTONG BIOCHEM ENG
Synthesis method of N-{2-[4-(2-pyrimidyl)-1-piperazine}adamantine-1-formide
InactiveCN102180867AEasy to routeEasy to operateOrganic chemistrySynthesis methodsStructural formula
The invention discloses a synthesis method of N-{2-[4-(2-pyrimidyl)-1-piperazine} adamantane-1-formide. The structural formula of the N-{2-[4-(2-pyrimidyl)-1-piperazine} adamantine-1-formide is shown in the specification. In the synthesis method, based on 2-chloropyrimidine as a main raw material, an adamantine-containing antidepressant N-{2-[4-(2-pyrimidyl)-1-piperazine} adamantane-1-formide is synthesized through nitrogen alkylation of 2-chloropyrimidine and anhydrous piperazine, alkylation of another nitrogen atom on the piperazine and bromohydrocarbon, Gabriel hydrazinolysis and amidation. The synthesis route in the invention is simple and practicable, raw material sources are abundant, reaction steps are less, and the yield of the product is high.
Owner:GUANGDONG UNIV OF TECH
A kind of synthetic method of antitumor drug
ActiveCN104817541BHigh yieldMild reaction conditionsCarbamic acid derivatives preparationOrganic compound preparationCarbamateMethyl-1H-indole
The invention relates to a synthetic method of an anti-tumor medicine, namely N-[2-[[2-(dimethylamino) ethyl] methyl amino]-4-methoxy-5-[[4-(1-methyl-1H-indole-3-yl)-2-pyrimidyl] amino] phenyl]-2-acrylamide (AZD9291) and a key intermediate of the anti-tumor medicine. The synthetic method comprises the following steps: performing Boc acid anhydride protection on 4-fluoro-2-methoxy-5-nitroaniline to obtain 4-fluoro-2-methoxy-5-nitroanilino tert-butyl formate, then reacting with N,N,N'-trimethylethylenediamine to obtain 4-(N,N,N'-trimethylethylenediamino)-2-methoxy-5-nitroanilino tert-butyl formate, then reducing to obtain 2-(N,N,N'-trimethylethylenediamino)-4-methoxy-5-tert-butyl carbamate phenylamine, then completely reacting with acryloyl chloride and directly removing a Boc protecting group to obtain 2-methoxy-4-N,N,N'-trimethylethylenediamino-5-acrylamido phenylamine, and finally reacting with 3-(2-chloropyrimidine-4-yl)-1-methylindole to obtain AZD9291. A process disclosed by the invention is simple in step, relatively high in yield, mild in reaction condition and easy for realization of industrial production.
Owner:苏州东南药业股份有限公司
Preparation method of hexachloroacetone
ActiveCN109942392AHigh yieldLow impurity contentOrganic compound preparationCarbonyl compound preparationImpurityAcetone
The invention relates to a preparation method of hexachloroacetone, belonging to the technical field of the preparation of organic chemical gas. The preparation method comprises the following steps: mixing a compound A with a chlorine molecule B, and reacting at 30-150 DEG C under the effect of a catalyst, wherein a reaction product is a mixture containing hexachloroacetone and the catalyst; and separating the catalyst from the mixture, so as to obtain a crude product; purifying the crude product, so as to obtain hexachloroacetone, wherein the compound A is at least one of acetone and chloroacetone with the chlorine atomic number of 1-5, the chlorine molecule B is chlorine or a mixture of chlorine and diluent gas, the diluent gas is inert gas or nitrogen, and the catalyst is pyrimidine, 2-chloropyrimidine or symtriazine. The method has the beneficial effects that the catalyst is easily recycled, and hexachloroacetone with extremely low impurity content can be obtained in a high-yield manner.
Owner:中国船舶集团有限公司第七一八研究所
Ionic iridium complex with pyrimidine ligand and preparation method thereof
InactiveCN104177439AChange the emission peak positionImprove transfer efficiencyGroup 8/9/10/18 element organic compoundsLuminescent compositionsEnergy transferIon exchange
The invention provides an ionic iridium complex with pyrimidine ligand and a preparation method thereof. The iridium complex has a chemical formula as follows. The preparation method of the ionic iridium complex is as below: first reacting substituted 2-chloro pyrimidine or 4,6-dimethyl pyrimidine with borophenylic acid or 2, 4-difluoro borophenylic acid to prepare a ligand; reacting the ligand with a compound iridous chloride (IrCl3.3H2O) to obtain chlorine bridge; and reacting the chlorine bridge with bisglyoxaline and conducting ion exchange reaction with salt to obtain the complex. Using iridium complex with pyrimidine derivative as an electrophosphorescent material has the following characteristics: for a same device, the pyrimidine-type iridium complex device has longer service life than a 2-phenylpyridine device; and the N atom electron cloud in the complex is more prominent, so as to increase the interaction with other molecules and improve the efficiency of energy transfer.
Owner:NINGBO UNIV
Method for preparing 2-chloropyrimidine
The invention discloses a method for preparing 2-chloropyrimidine, belonging to the technical field of methods for preparing chemical medicine intermediates. The method for preparing the 2-chloropyrimidine comprises the following steps of: (1) reacting dicyandiamide with ammonium chloride at high temperature, and obtaining guanidine hydrochloride; (2) reacting the guanidine hydrochloride with 1.1.3.3-tetramethoxypropane by refluxing under the condition of taking industrial hydrochloric acid as a solvent to obtain 2-amino pyrimidine; and (3) reacting the 2-amino pyrimidine with sodium nitrate at low temperature for producing the 2-chloropyrimidine by taking zinc chloride as a catalyst and taking the industrial hydrochloric acid as the solvent. The preparation method disclosed by the invention has the advantages of lower cost of raw materials, relatively simple technical process, mild reaction conditions, low operation difficulty, excellent maneuverability; convenience for post-treatment; and largely improved yield stable quality and applicability of large-scale industrial production of prepared products,.
Owner:LINHAI TIANYU PHARMA
Synthetic C2-acetoxy-3-indolone compound and method
The invention discloses a synthetic C2-acetoxy-3-indolone compound and a method thereof. The method comprises the following steps: using indole compounds as raw materials; carrying out a reaction between indole or substituent indole with 2-chloropyrimidine under alkaline conditions of sodium hydride to generate 1-(pyrimidinyl-2-yl)-1H-indole compound; introducing a pyrimidine directing group into the indole compound; allowing 1-(pyrimidinyl-2-yl)-1H-indole compound to participate in an oxidation reaction in the presence of iodosobenzene diacetate as an oxidant and a mixed solvent of acetic acid and acetic anhydride; finally synthesizing the C2-acetoxy-3-indolone compound. As an indole structure is directly used in an oxidizing reaction, a pre-functionalization process is eliminated to reduce reaction steps, the reaction economic efficiency is improved, and the C2-acetoxy-3-indolone compound is synthesized effectively under a relatively mild condition. The structural unit can be used as pharmaceutical intermediate, polymer substrate, premise of total synthesis, and has a wide biological activity and a broad application prospect.
Owner:NANJING UNIV OF SCI & TECH
Green preparation method of pazopanib hydrochloride
The invention belongs to the technical field of pharmaceutical chemistry synthesis and particularly relates to a green preparation method of pazopanib hydrochloride. The method comprises the steps ofallowing o-toluidine and N-chlorosuccinimide to give a chlorination reaction to form 2-methyl-5-chlorine-aniline, allowing 2-methyl-5-chlorine-aniline to react with a nitrous acid compound to form 6-chlorine-2H-indole hydrochloride, performing N methylation reaction to form N-methyl-6-chlorine-2H-indole, performing 3-delta carbon alkylation reaction in the presence of dimethyl sulfoxide to form 2,3-dimethyl-6-chlorine-2H-indazole, allowing 2,3-dimethyl-6-chlorine-2H-indazole to react with 2-chlorine-4-amino-pyrimidine and methyl iodide to form N-(2-chloropyrimidine-4)-N-methyl-2,3-dimethyl-2H-indazole-6-amine, and at last, allowing N-(2-chloropyrimidine-4)-N-methyl-2,3-dimethyl-2H-indazole-6-amine to react with 3-sulfamate-4-methyl-aniline to form pazopanib hydrochloride. The method is lowin raw material price, simple to operate and low in operational risk, and avoids generation of waste acid; and a reaction yield and purity are high.
Owner:JINAN ASIA PHARMA TECH
Imino compounds with a 2-chloropyrimidin-5-yl substituent as pest-control agents
Provided herein are imino compounds of formula Iand stereoisomers, tautomers and salts thereof. Further provided herein are agricultural and veterinary compositions including the compounds. Also provided herein are methods related to the use of these compounds, and the stereoisomers, tautomers and / or salts thereof, for combating invertebrate pests. Further provided herein are methods for combating invertebrate pests, wherein the methods include applying such compounds, steroisomers, tautomers, and salts.
Owner:BASF AG
Method for preparing rosuvastatin calcium
ActiveCN102219749BLow priceHigh yieldOrganic chemistryBulk chemical productionRosuvastatin CalciumProtecting group
The invention provides a preparation method of rosuvastatin calcium. The method comprises the following steps: carrying out condensation reaction on 4-(4-fluorophenyl)-6-isopropyl-2 chloropyrimidine-5-formaldehyde and (3R)-tertiary butyl dimethylsiloxo-5-oxo-6-triphenyl phosphaalkene hexanoate; and then removing a silylether protecting group of hydroxyl, carrying out stereoselectivity reduction, reacting with 2,2-dimethoxy propane, condensing with N-methyl methane sulfonamide, removing propylidene protection, hydrolyzing an ester group, salifying and carrying out other steps, so as to obtain the rosuvastatin calcium. According to the preparation method, the yield is stable, and the price of reagents is cheap; and the method is easy to operate and is beneficial to industrial production.
Owner:ZHEJIANG JINGXIN PHARMA
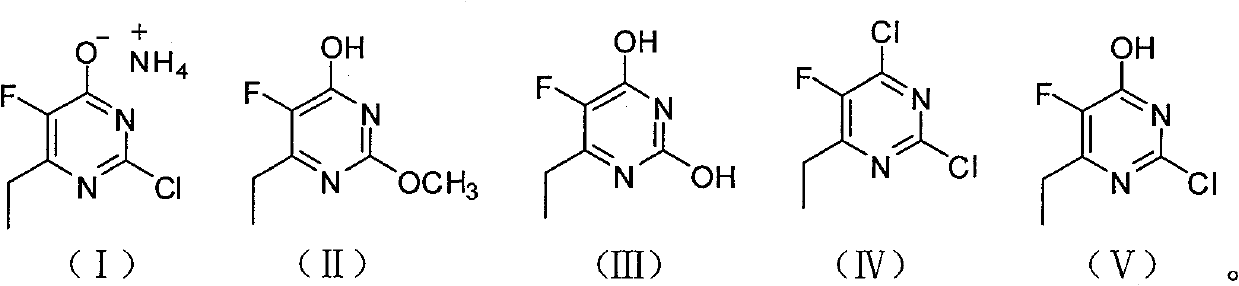
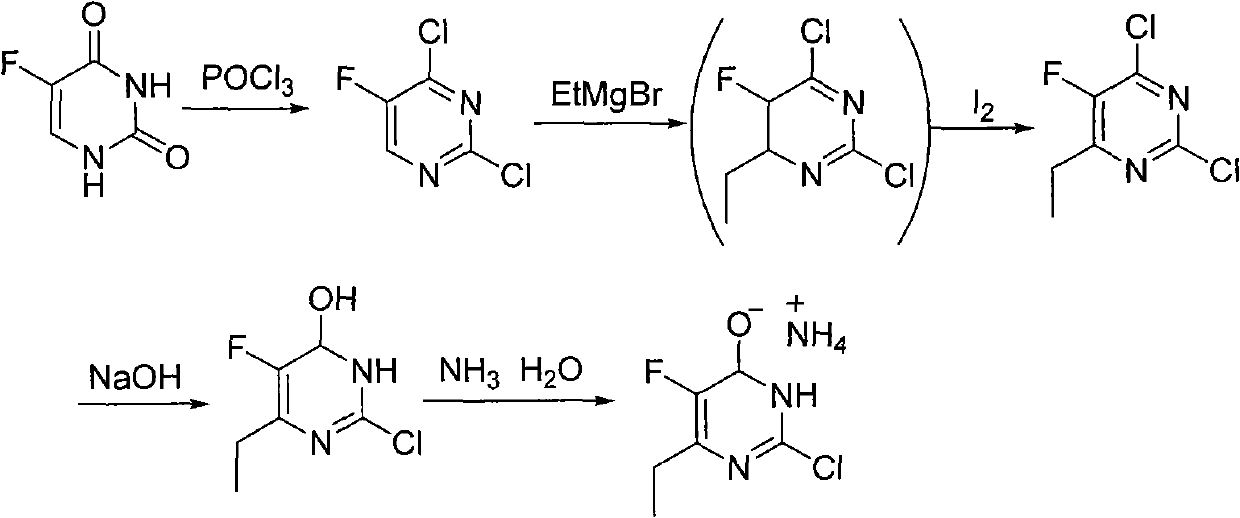
Synthesis method of 6-ethyl-4-hydroxyl-5-fluorine-2-cloro pyridine ammonium salt
The invention discloses a synthesis method of 6-ethyl-4-hydroxyl-5-fluorine-2-cloro pyridine ammonium salt with a structure showed as a formula (I), comprising the following steps: taking 2-fluorine-3-oxo valerate as a starting material, and condensing with O-methylisourea in alcoholic solution of sodium alcoholate to prepare an intermediate (II); carrying out acid-catalyzed hydrolysis on the intermediate (II) to prepare an intermediate (III); carrying out chlorinated reaction on the intermediate (III) by adopting chlorinated reagent to prepare a compound (IV); and preparing an intermediate (V) by the compound (IV) in alkaline water solution through hydroxyl substitution, and ammoniating the intermediat (V) by ammonia water to form salt, namely, obtaining the 6-ethyl-4-hydroxyl-5-fluorine-2-cloro pyridine ammonium salt. The synthesis method adopts cheap and easily-obtained starting raw material, avoids the use of format reagent and iodine, simplifies operations, reduces environmentalpollution, is beneficial to industrial production and has lower production cost and good product quality.
Owner:上海永阔生物医药科技有限公司
A method for synthesizing 2,2'-dipolybenzazole compounds
The invention discloses a method for synthesizing 2,2'-dindolylmethane compounds. Firstly, 1-(pyrimidinyl-2-yl)-1H-indole compounds are generated in the mode that indole or substituded indole reacts with 2-chloropyrimidine under the sodium hydride alkaline condition with indole compounds as raw materials, indole guiding groups are introduced into the indole compounds, then the 1-(pyrimidinyl-2-base)-1H-indole compounds are subjected to a dimerization reaction under the action of acetic acid copper, silver nitrate and a toluene solvent, and finally the 2,2'-dindolylmethane compounds are synthesized. A direct carbon activation reaction method is applied, reaction steps are reduced, economical efficiency of reaction is improved, the target product 2,2'-dindolylmethane compounds are efficiently synthesized under the mild condition, indole groups can be removed after the 2,2'-dindolylmethane compounds are de-protected, 2,2'-dindolylmethane is generated, and the structural unit has wide biological activity and application prospects.
Owner:NANJING UNIV OF SCI & TECH
Preparation method of piribedil
The invention relates to a preparation method of piribedil. The method is characterized in that piperidine is used as a starting raw material, and piperonyl chlorine is prepared through a Blanc reaction; in addition, 2-cloro pyridine is used as a starting raw material to carry out single alkylation on piperazine to obtain 1-(2-pyrimidyl)piperazine; and the prepared piperonyl chlorine and the 1-(2-pyrimidyl)piperazine are subjected to an N-alkylation reaction to obtain the piribedil. The invention has the advantages that the raw material piperidine has low price and is easy to obtain; the reaction condition is moderate, and reactions of all steps can be quickly carried out at lower temperature without high temperature, high pressure or special catalysts; the yield of products is higher; a purification method is simple; the purity is better (the purity content of HPLC is not lower than 99.8 percent), and the like, thus the method is a route which has simple operation, higher yield, lower cost and stable quality and is suitable for industrial production.
Owner:KANGYA OF NINGXIA PHARMA
Method for synthesizing 2-chloropyrimidine
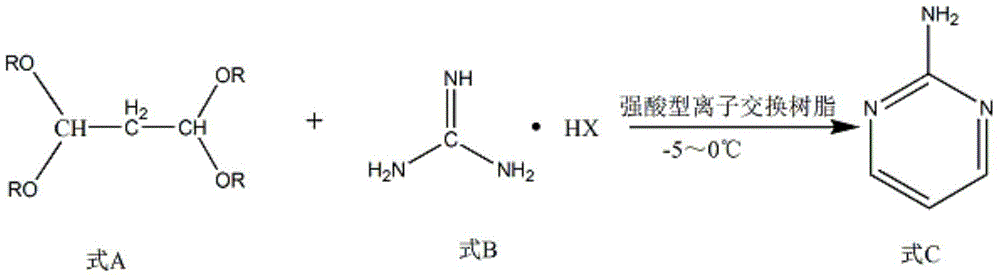
The invention discloses a method for preparing 2-chloropyrimidine. According to the method, firstly, tetraalkoxy malonaldehyde and guanidine salt have a cyclization reaction in a polar solvent at the temperature of subzero 5 DEG C-0 DEG C under the catalysis of strong acid action exchange resin serving as a catalyst, pH of a product obtained through the cyclization reaction is adjusted to 7-8, reduced pressure distillation is performed, 2-aminopyrimidine is obtained and is reduced at the temperature of subzero 10 DEG C-5 DEG C under the catalysis of NaNO2, HCl and ZnCl2 serving as catalysts, and a 2-chloropyrimidine product is obtained. By means of the synthetic route, 2-chloropyrimidine with higher purity and higher yield is obtained.
Owner:TAICANG YUNTONG BIOCHEM ENG
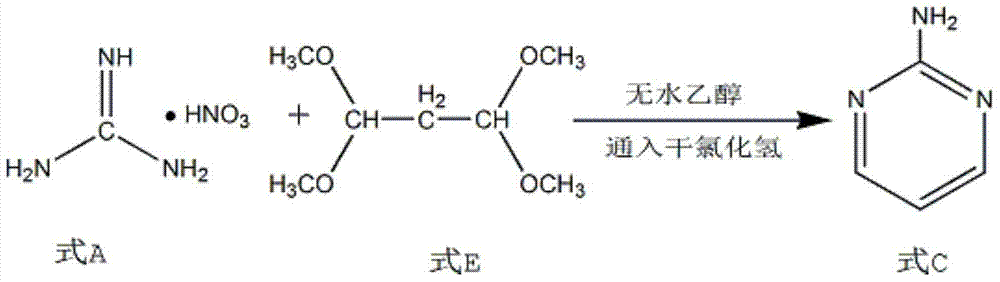
Synthesis process of 2-chloropyrimidin
The invention discloses a synthesis process of 2-chloropyrimidine. The synthesis method of the invention is as below: reacting guanidine nitrate and 1,1,3,3-tetramethoxy propane in an absolute ethyl alcohol solvent with continuous introduction of dry hydrogen chloride gas at 20-30 DEG C for 4-6 h to obtain 2-aminopyrimidine; and then reducing 2-aminopyrimidine in the presence of catalysts of NaNO2, HCl and ZnCl2 at -10-5 DEG C to obtain the product. Through the above preparation route, 2-chloropyrimidine with high purity and high yield can be obtained.
Owner:TAICANG YUNTONG BIOCHEM ENG
Method for synthesizing 2,2'-dindolylmethane compounds
The invention discloses a method for synthesizing 2,2'-dindolylmethane compounds. Firstly, 1-(pyrimidinyl-2-yl)-1H-indole compounds are generated in the mode that indole or substituded indole reacts with 2-chloropyrimidine under the sodium hydride alkaline condition with indole compounds as raw materials, indole guiding groups are introduced into the indole compounds, then the 1-(pyrimidinyl-2-base)-1H-indole compounds are subjected to a dimerization reaction under the action of acetic acid copper, silver nitrate and a toluene solvent, and finally the 2,2'-dindolylmethane compounds are synthesized. A direct carbon activation reaction method is applied, reaction steps are reduced, economical efficiency of reaction is improved, the target product 2,2'-dindolylmethane compounds are efficiently synthesized under the mild condition, indole groups can be removed after the 2,2'-dindolylmethane compounds are de-protected, 2,2'-dindolylmethane is generated, and the structural unit has wide biological activity and application prospects.
Owner:NANJING UNIV OF SCI & TECH
Process for synthesizing 2-chloro-pyrimidine
The invention discloses a process for synthesizing 2-chloro-pyrimidine. The process disclosed by the invention comprises the following steps: firstly, dissolving dicyandiamide into sulfuric acid at 2-6 DEG C, heating at 50-60 DEG C, and carrying out hydrolysis reaction for 4-6 hours, so as to obtain guanidine sulfate; reacting with propargyl ethyl alcohol at 50-55 DEG C for 8-12 hours, so as to obtain 2-aminopyrimidine; and finally restoring the 2-aminopyrimidine at -10 DEG C to 5 DEG C by taking NaNO2, HCl and ZnCl2 as catalysts, so as to obtain the product. Through the synthesis route, the 2-chloro-pyrimidine with relatively high purity and relatively high yield is obtained.
Owner:TAICANG YUNTONG BIOCHEM ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com