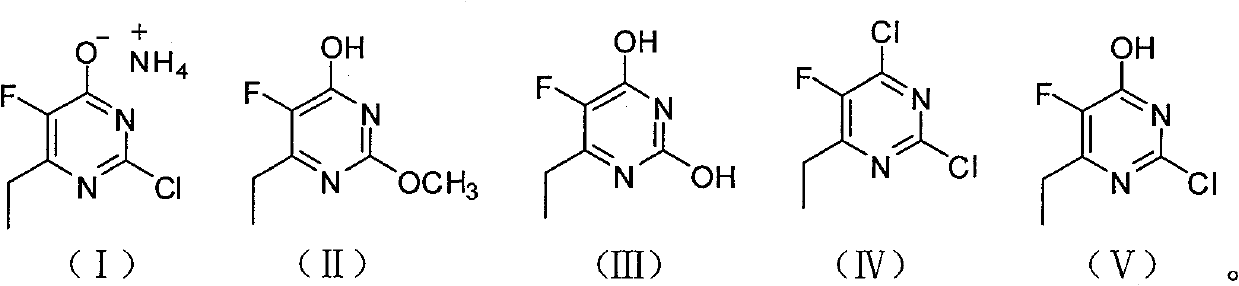
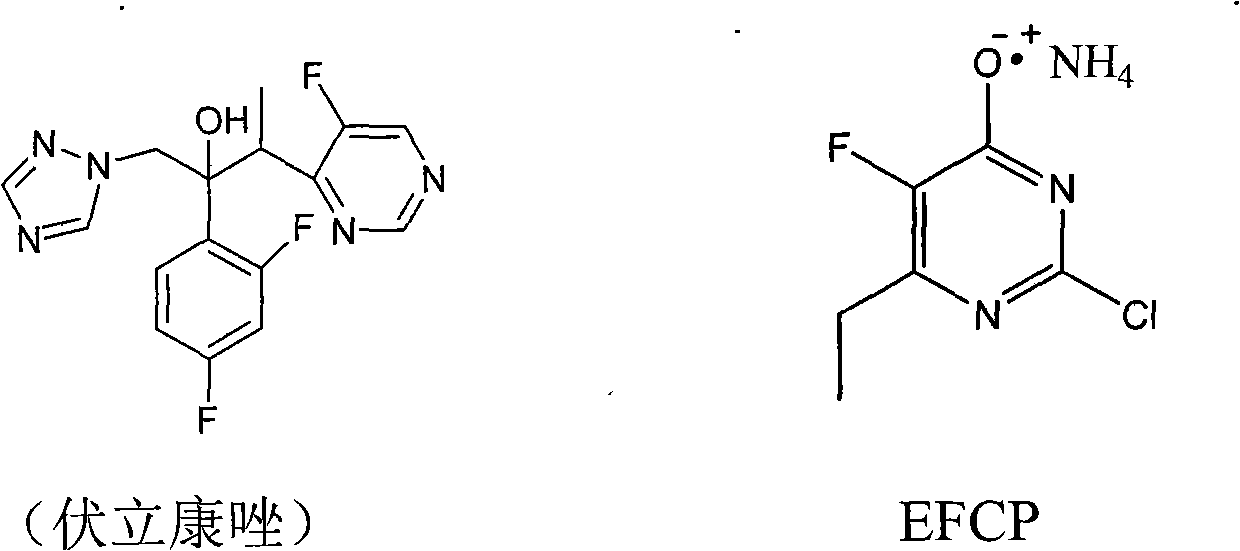
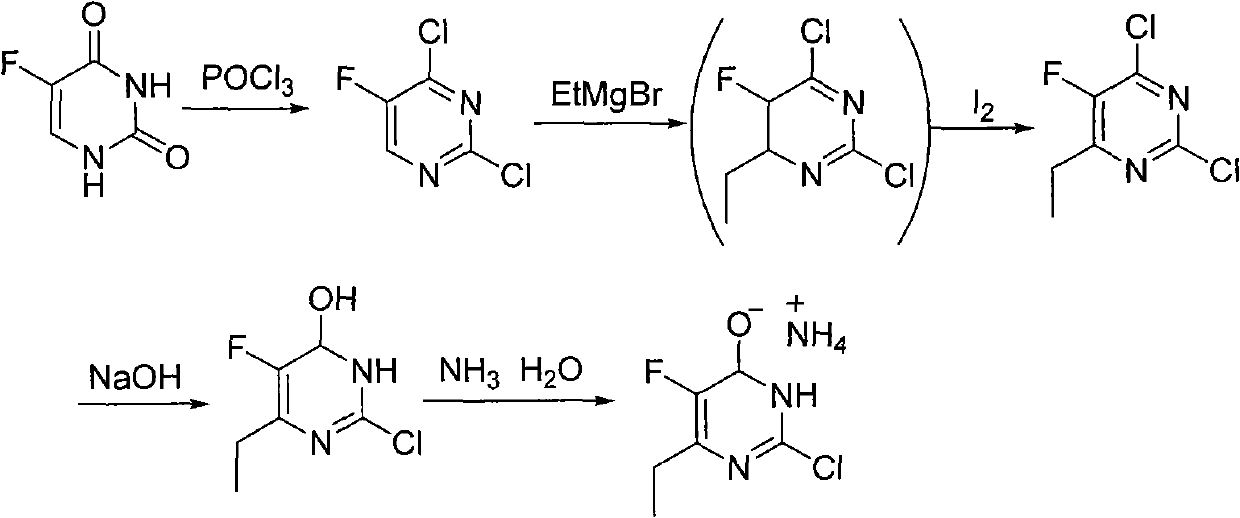
Synthesis method of 6-ethyl-4-hydroxyl-5-fluorine-2-cloro pyridine ammonium salt
A technology of chloropyrimidine ammonium salt and synthesis method, which is applied in the field of synthesis of 6-ethyl-4-hydroxy-5-fluoro-2-chloropyrimidine ammonium salt, and can solve the problem of poor oxidation effect compared to iodine, low yield waste liquid, etc. problems, to avoid the use of format reagents and iodine, good product quality, and low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0038] Example 1.2 Condensation reaction
[0039] Add 160ml methanol to a 250ml reaction flask, add 9.3g (0.405mol) sodium metal in batches with stirring. After the sodium reaction is completed, the reaction solution is cooled to about 5℃ with an ice water bath, and then 42g (0.203mol) O are added separately -Methylisourea sulfuric acid monomethyl salt and 20g (0.135mol) 2-fluoro-3-oxopentanoic acid methyl ester, after the addition, keep it under 25℃ for half an hour, then heat to reflux (65℃)3 hour. The reaction was stopped and the methanol was evaporated under reduced pressure. The residue was dissolved in 200ml of water. The pH value was adjusted to about 3 with 6M HCl. A solid was precipitated. After cooling in an ice water bath for 2 hours, it was filtered. The resulting mother liquor was concentrated again to obtain a total of 9.7g The intermediate (II) is a white powder with a weight yield of 48.5%.
Embodiment 13
[0040] Example 1.3 Condensation reaction
[0041] Add 160ml methanol into a 250ml reaction flask, add 6.2g (0.270mol) sodium metal in batches with stirring. After the sodium reaction is completed, the reaction solution is cooled to about 5℃ in an ice water bath, and then 84g (0.406mol) O are added separately -Methylisourea sulfuric acid monomethyl salt and 20g (0.135mol) 2-fluoro-3-oxopentanoic acid methyl ester, after the addition, keep the temperature below 25°C for half an hour, and then heat and reflux for 3 hours. The reaction was stopped and the methanol was evaporated under reduced pressure. The residue was dissolved in 200ml of water. The pH was adjusted to about 3 with 6M HCl. A solid was precipitated. After cooling in an ice-water bath for 2 hours, it was filtered. The mother liquor was concentrated again to obtain 10.3g. The intermediate (II) has a weight yield of 51.5%.
Embodiment 14
[0042] Example 1.4 Condensation reaction
[0043] Add 160ml methanol into a 250ml reaction flask, add 6.2g (0.270mol) sodium metal in batches with stirring, after the sodium reaction is completed, the reaction solution is cooled to about 5℃ with an ice water bath, and then 28g (0.135mol) O are added separately -Methylisourea sulfate monomethyl salt and 20g (0.135mol) 2-fluoro-3-oxopentanoic acid methyl ester, after the addition, the reaction is maintained at 35°C for 6 hours. The reaction was stopped and the methanol was evaporated under reduced pressure. The residue was dissolved in 200ml of water. The pH was adjusted to about 3 with 6M HCl. A solid was precipitated. After cooling in an ice-water bath for 2 hours, it was filtered. The resulting mother liquor was concentrated again to obtain a total of 8.5g The intermediate (II) has a weight yield of 42.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com