Process for synthesizing 2-chloro-pyrimidine
A kind of chloropyrimidine and process technology, applied in the field of 2-chloropyrimidine preparation, can solve the problems of low yield, low product purity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
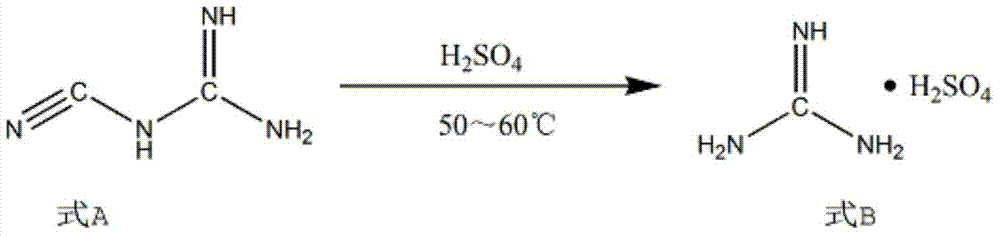
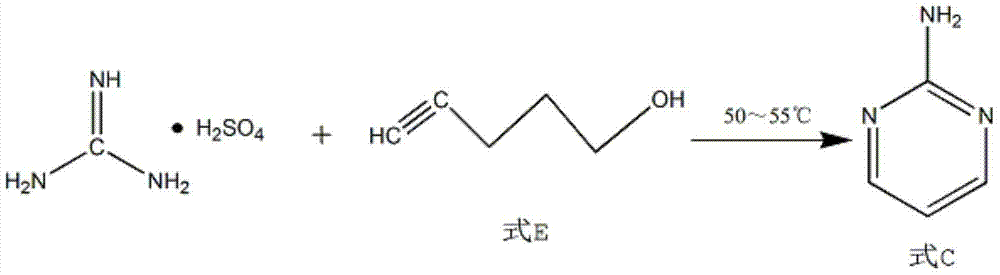
[0018] Add 1.5 mol of solute and 96 wt% sulfuric acid into a 250ml four-necked flask, adjust its temperature to 2°C, slowly add 1mol of dicyandiamide to the four-necked flask while stirring constantly, and raise the temperature to 50°C to make it Hydrolysis reaction 6h. Then cool to room temperature, add 1 / 3 mol propargyl ethanol to the four-necked flask, heat up to 50°C for cyclization reaction for 12 hours, filter the product of the cyclization reaction, wash with water and adjust the pH to 8 with sodium hydroxide for use Extract with isobutanol to obtain the product 2-aminopyrimidine.
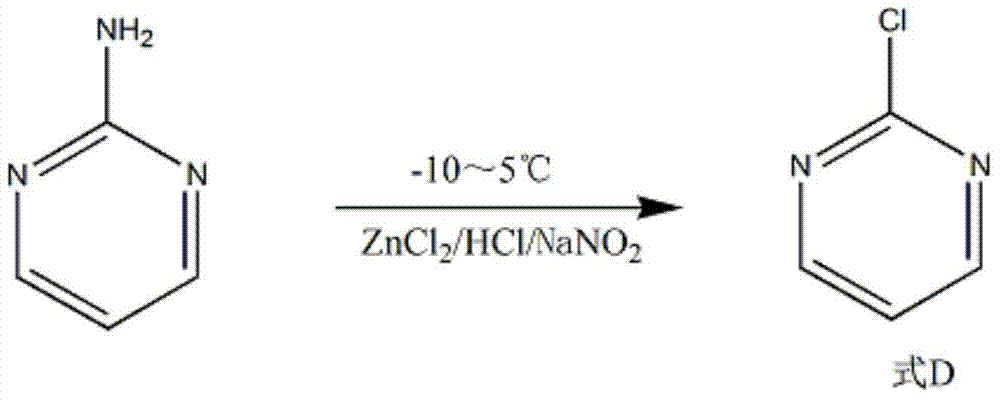
[0019] Add 2.5 mol of hydrochloric acid and 80 g of dichloromethane into a 500 mL reactor, and then add 1 mol of 2-aminopyrimidine under stirring. Add 3.2mol ZnCl at 10~15℃ 2 , After reacting for 30min, a dark yellow suspension was generated. Then it was cooled to -10°C, and 2.4 mol of sodium nitrite was added slowly within 4 hours under the protection of nitrogen. After continuing to fe...
Embodiment 2
[0021] Add a 250ml four-necked flask to 6mol of solute and 94wt% mass concentration of sulfuric acid, adjust its temperature to 6°C, slowly add 1mol of dicyandiamide to the four-necked flask while stirring continuously, and heat up to 60°C to hydrolyze it Reaction 4h. Then cool to room temperature, add 1 mol propargyl ethanol to the four-necked flask, heat up to 55°C for cyclization reaction for 8 hours, filter the product of the cyclization reaction, wash with water and adjust the pH to 9 by sodium hydroxide. Alcohol is extracted to obtain the product 2-aminopyrimidine.
[0022] Add 3.5 mol of hydrochloric acid and 80 g of dichloromethane into a 500 mL reactor, and then add 1 mol of 2-aminopyrimidine under stirring. Add 4mol ZnCl at 10~15℃ 2 , After reacting for 60min, a dark yellow suspension was generated. Then cool to 5°C, and add 2.8mol sodium nitrite within 3h under the protection of nitrogen. After continuing to feed nitrogen for 0.5 h, the reaction was terminated. ...
Embodiment 3
[0024] Add 250ml four-necked flask to sulfuric acid with 3mol of solute and 95wt% mass concentration, adjust its temperature to 5°C, slowly add 1mol of dicyandiamide into the four-necked flask while stirring continuously, and heat up to 55°C to hydrolyze it Reaction 5h. Then cool to room temperature, add 0.418mol propargyl ethanol to the four-necked flask, heat up to 53°C for cyclization reaction for 10h, filter the product of the cyclization reaction, wash with water, and adjust the pH to 8.5 with sodium hydroxide. Butanol is extracted to obtain the product 2-aminopyrimidine.
[0025] Add 3 mol of hydrochloric acid and 80 g of dichloromethane into a 500 mL reactor, and then add 1 mol of 2-aminopyrimidine under stirring. Add 3.5mol ZnCl at 10~15℃ 2 , After reacting for 45min, a dark yellow suspension was generated. Then cool to 0°C, and add 2.5mol sodium nitrite within 3.5h under the protection of nitrogen. After continuing to feed nitrogen for 40 min, the reactant was pou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com