Method for synthesizing 2,2'-dindolylmethane compounds
A technology for dipolybenzazole and compound, which is applied in the field of synthesizing 2,2'-dipolybenzazole compounds, can solve the problems of only 35-55% yield, limited sources and low economy, etc. The effect of high economy, wide biological activity and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
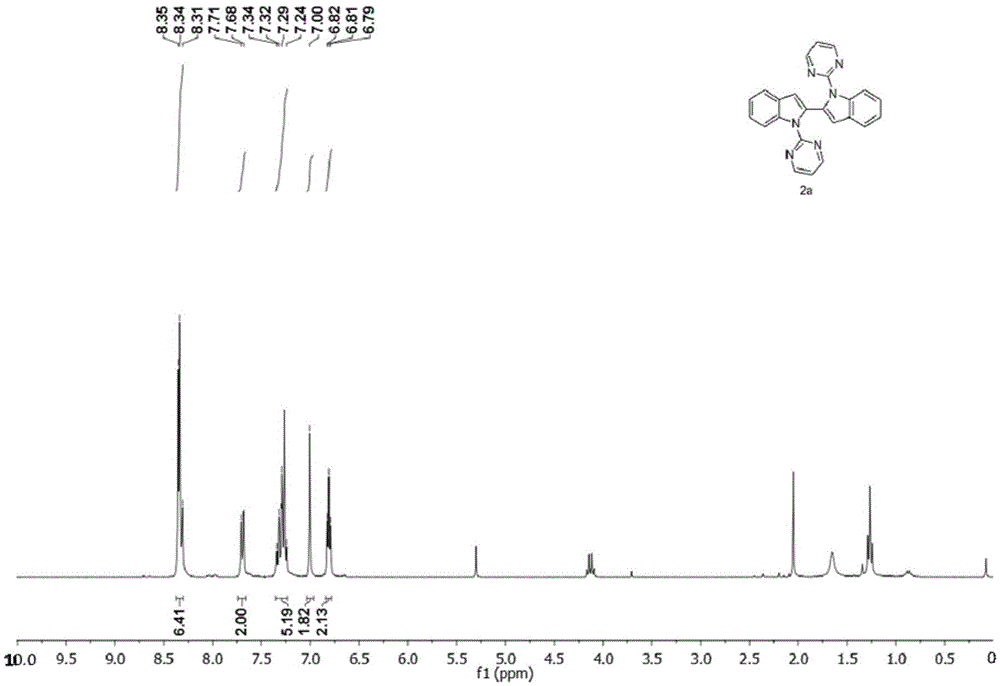
[0037] Synthesis of 1,1'-bis(pyrimidinyl-2-yl)-1H,1'H-2,2'-diindole
[0038]
[0039] In the reaction vessel, add indole (6mmol, 702.9mg), NaH (mass fraction 60%, 10.8mmol, 432mg), DMF solvent, and react under low temperature for 30 minutes; then add 2-chloropyrimidine (9.6mmol, 1099.488mg ), the mixture was reacted at 100°C for 24 hours, cooled to room temperature, extracted to remove the solvent, and separated by column to obtain compound 1-(pyrimidinyl-2-yl)-1H-indole reactant. Accurately weigh the 1-(pyrimidinyl-2-yl)-1H-indole reactant (0.2mmol, 39.16mg) with an electronic balance, transfer to the reaction vessel, and add AgNO to the reaction vessel 3 (0.20mmol, 33.98mg), Cu(OAc) 2(0.2mmol, 39.90mg), dropwise added toluene solvent into the reaction tube, tightened the stopper of the reaction tube to seal the reaction system, heated to 130°C, and stirred for 16h. After the reaction, the reaction solution was cooled to room temperature, and the solvent was removed unde...
Embodiment 2
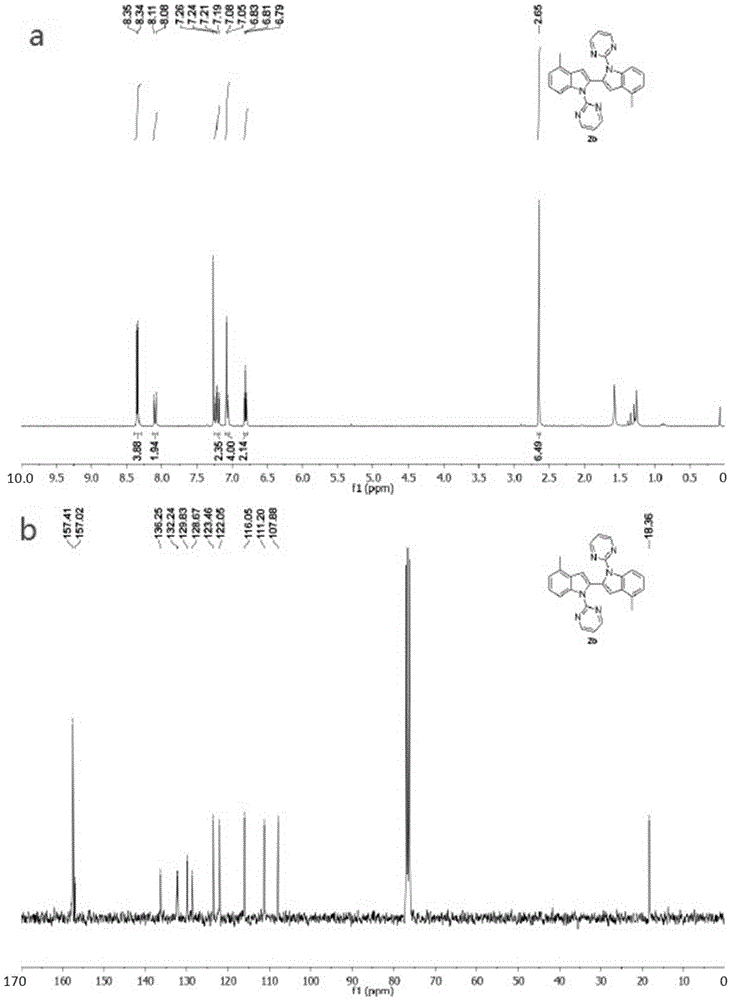
[0042] Synthesis of 4,4'-dimethyl-1,1'-bis(pyrimidinyl-2-yl)-1H,1'H-2,2'-diindole.
[0043]
[0044] In the reaction vessel, add 4-methylindole (6mmol, 787.08mg), NaH (mass fraction 60%, 7.2mmol, 288mg), DMF solvent, react at low temperature for 60 minutes; then add 2-chloropyrimidine (6.6 mmol, 755.898 mg), the mixture was reacted at 130°C for 16 hours, cooled to room temperature, extracted to remove the solvent, and separated by column to obtain compound 4-methyl-1-(pyrimidinyl-2-yl)-1H-indole reaction thing. Accurately weigh 4-methyl-1-(pyrimidinyl-2-yl)-1H-indole reactant (0.2mmol, 41.85mg) with an electronic balance, transfer to the reaction vessel, add AgNO to the reaction vessel 3 (0.28mmol, 47.57mg), Cu(OAc) 2 (0.3mmol, 59.85mg), dropwise added toluene solvent into the reaction tube, sealed the reaction system, heated to 110°C, and stirred for 24h. After the reaction, the reaction solution was cooled to room temperature, and the solvent was removed under vacuum t...
Embodiment 3
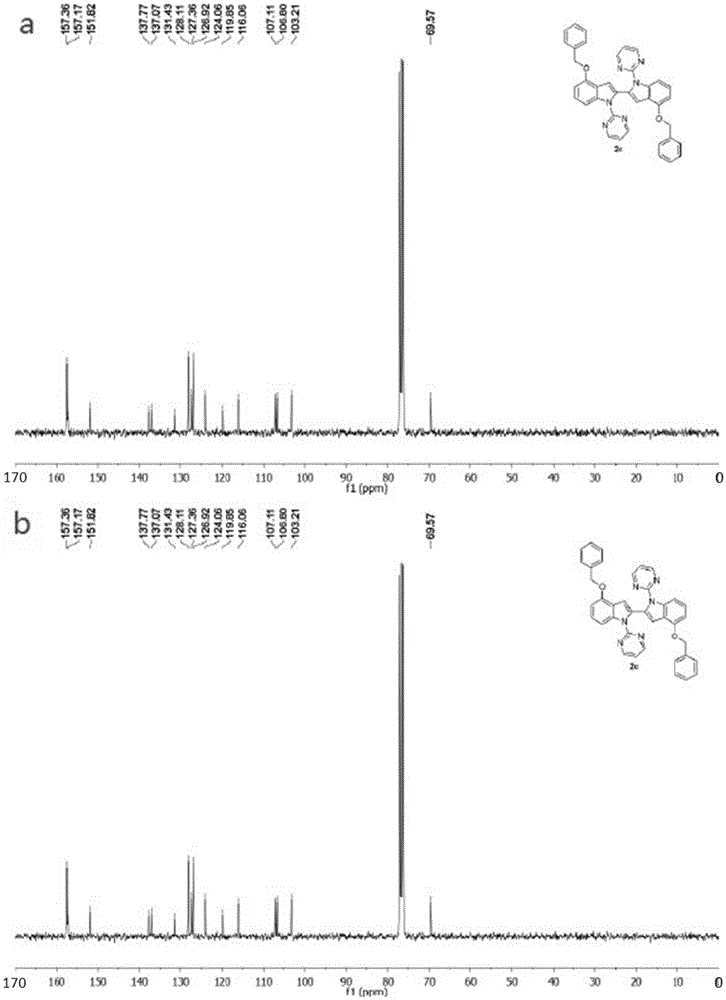
[0047] Synthesis of 4,4'-dibenzyloxy-1,1'-bis(pyrimidinyl-2-yl)-1H,1'H-2,2'-diindole.
[0048]
[0049] In the reaction vessel, add 4-benzyloxyindole (6mmol, 1339.68mg), NaH (mass fraction 60%, 9mmol, 360mg), DMF solvent, react under low temperature condition for 40 minutes; Then add 2-chloropyrimidine (7.2 mmol, 824.616g), the mixture was reacted at 130°C for 24 hours, cooled to room temperature, extracted to remove the solvent, and separated by column to obtain compound 4-benzyloxy-1-(pyrimidinyl-2-yl)-1H-indole Reactant. Accurately weigh 4-benzyloxy-1-(pyrimidinyl-2-yl)-1H-indole reactant (0.2mmol, 60.27mg) with an electronic balance, transfer to the reaction vessel, add AgNO to the reaction vessel 3 (0.24mmol, 40.77mg), Cu(OAc) 2 (0.2mmol, 39.90mg), dropwise added toluene solvent into the reaction tube, sealed the reaction system, heated to 130°C, and stirred for 24h. After the reaction, the reaction solution was cooled to room temperature, and the solvent was remove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com