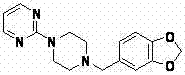
Preparation method of piribedil
A technology of piribedil and molar ratio, applied in the field of preparation of piribedil, can solve the problems of large amount of ruthenium reagent, high yield, low yield, etc., achieve mild reaction conditions, simple purification method, and product yield high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
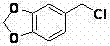
[0041] 1. Preparation of Piperonyl Chloride
[0042] In a 1L three-neck flask, add piperonylcycline (200g, 1.64mol) and paraformaldehyde (78.5g, 2.61mol), stir vigorously at 25°C, add concentrated hydrochloric acid (532ml) dropwise, and continue stirring after about 1h After 4 hours, the organic layer was separated to obtain 246 g of a slightly turbid gray oil, yield: 88.0%.
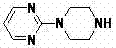
[0043] 2. Preparation of 1-(2-pyrimidinyl)piperazine
[0044]In a 2L three-necked flask, add anhydrous piperazine (375.5g, 4.37mol) and absolute ethanol (400ml), and stir vigorously at 50°C; dissolve 2-chloropyrimidine (100g, 0.87mol), slowly added dropwise to the above reaction flask, after about 2.5h, continue to stir for 1h, concentrate to dryness under reduced pressure, add water (300ml), filter with suction, and extract the filtrate with chloroform (300ml×3). The water layer was allowed to stand still, crystals were precipitated, filtered by suction, and 260.0 g (wet weight) of piperazine hexahydr...
Embodiment 2
[0048] 1. Preparation of Piperonyl Chloride
[0049] With embodiment 1.
[0050] 2. Preparation of 1-(2-pyrimidinyl)piperazine
[0051] In a 250ml three-necked flask, add anhydrous piperazine (75.1g, 0.87mol) and water (80ml), and stir vigorously at 50°C; in addition, dissolve 2-chloropyrimidine (20g, 0.17mol ), slowly added dropwise to the above-mentioned reaction flask, after the addition, continued to stir for 1 h, concentrated under reduced pressure, filtered with suction, and the filtrate was extracted with chloroform (60ml×3). The water layer was allowed to stand still, crystals were precipitated, filtered by suction, and 49 g of piperazine hexahydrate (wet weight) was recovered; the organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain a light yellow oil 30.9g, yield: 107.9% (may contain some water).
[0052] 3. Preparation of piribedil
...
Embodiment 3
[0055] 1. Preparation of Piperonyl Chloride
[0056] With embodiment 1.
[0057] 2. Preparation of 1-(2-pyrimidinyl)piperazine
[0058] In a 250ml three-necked flask, add piperazine hexahydrate (169.4g, 0.87mol) and absolute ethanol (80ml), and stir vigorously at 50°C; in addition, dissolve 2-chloropyrimidine (20g, 0.17mol), slowly added dropwise to the above reaction flask, after the addition, continued to stir for 1h, concentrated to dryness under reduced pressure, added water (60ml), filtered with suction, and extracted the filtrate with chloroform (60ml×3). The water layer was left standing, crystals were precipitated, filtered by suction, and 60.1 g of piperazine hexahydrate (wet weight) was recovered; the organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain a light yellow oil Product 31.7g, yield: 110.7% (may contain some water).
[0059] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com