Synthetic C2-acetoxy-3-indolone compound and method
A kind of technology of acetoxy and compound, applied in the field of synthesizing C2-acetoxy-3-indolone compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
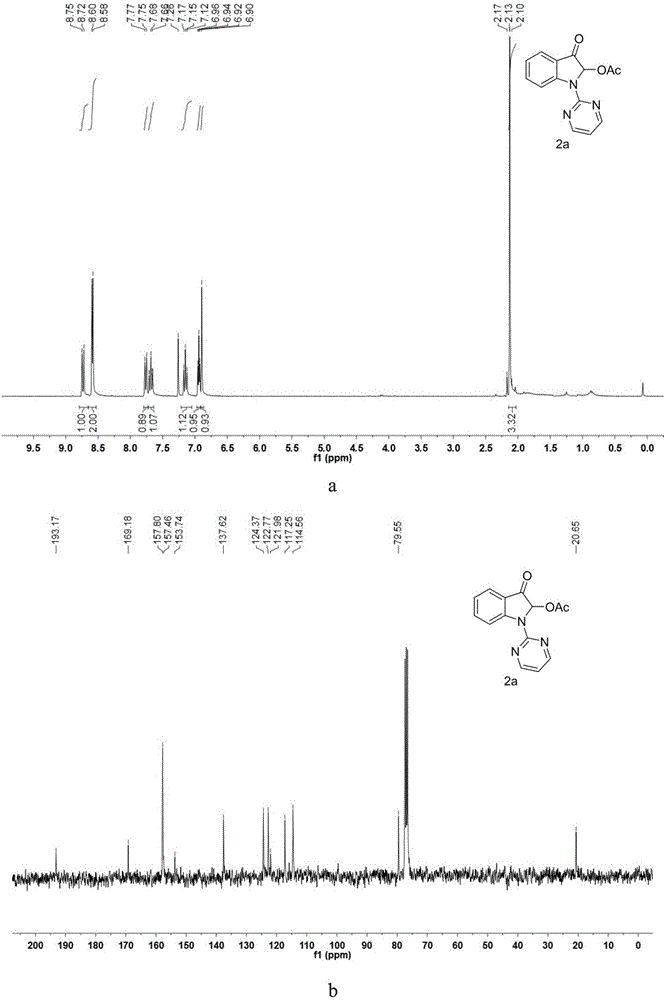
[0041] Synthesis of 3-oxo-2-acetoxy-1-(pyrimidin-2-yl)indole
[0042] In the reaction vessel, add indole (6mmol, 702.9mg), NaH (mass fraction 60%, 10.8mmol, 432mg), DMF solvent, and react under low temperature for 30 minutes; then add 2-chloropyrimidine (9.6mmol, 1099.488mg ), the mixture was reacted at 130° C. for 24 hours, cooled to room temperature, extracted to remove the solvent, and separated by column to obtain compound 1-(pyrimidinyl-2-yl)-1H-indole reactant. Accurately weigh the 1-(pyrimidinyl-2-yl)-1H-indole reactant (0.3mmol, 58.50mg) with an electronic balance of 1 / 10,000th, transfer to the thick-walled pressure-resistant tube of the reaction vessel, and add PhI to the reaction vessel (OAc) 2 (3eq, 289.5mg) was added dropwise 2mL of acetic acid / acetic anhydride = 7 / 3 mixed solvent, the reaction tube plug was tightened to seal the reaction system, heated to 60°C, and reacted for 36h under the condition of stirring in an oil bath. After the reaction was finished, t...
experiment example 2
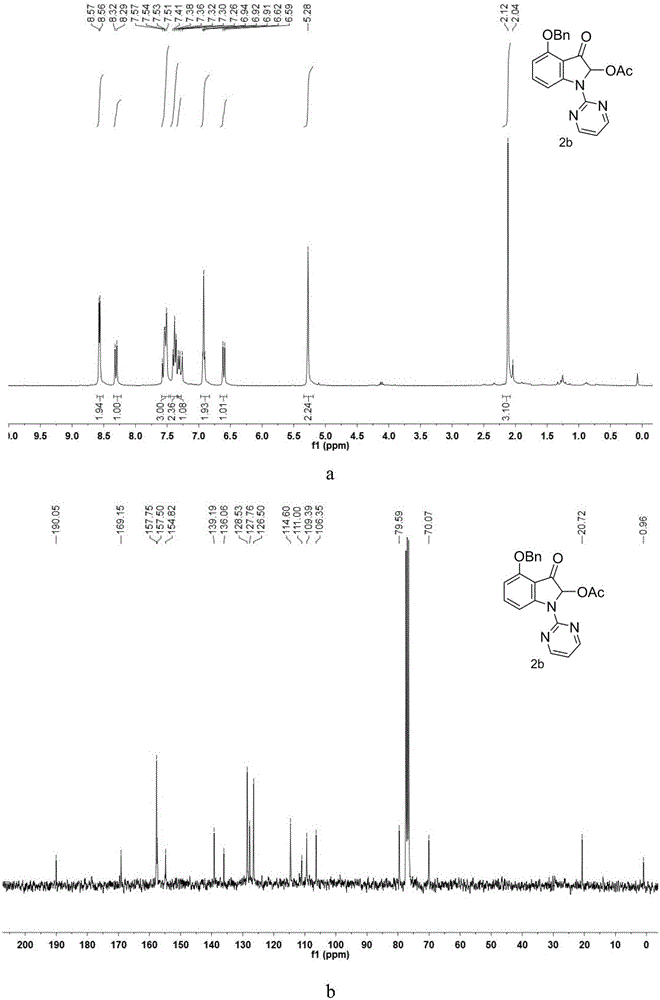
[0045] Synthesis of 4-(Benzyloxy)-2-Acetoxy-3-oxo-1-(pyrimidin-2-yl)indole
[0046] Take a 100mL round bottom flask and a suitable size magnet, weigh 4-(benzyloxy)-1H-indole (5mmol, 1115mg) and dissolve it in N,N-dimethylformamide (DMF), and place Slowly add NaH (mass fraction 60%, 7.5mmol, 300mg) into an ice-water bath for hydrogen extraction reaction, transfer to an oil bath at 130°C after 1 hour of reaction, monitor the end of the reaction by TLC after 24 hours, dilute with ethyl acetate and transfer to A 250mL separatory funnel was washed three times with water. After washing the DMF, the organic phase was collected to remove water with anhydrous sodium sulfate, and the organic solvent was removed by rotary evaporation under reduced pressure to obtain a crude product, which was passed through column chromatography (ethyl acetate:petroleum ether= 3:95) to give the product 4-(benzyloxy)-1H-(pyrimidin-2-yl)-1H-indole reactant. Accurately weigh the 4-(benzyloxy)-1H-(pyrimidin...
experiment example 3
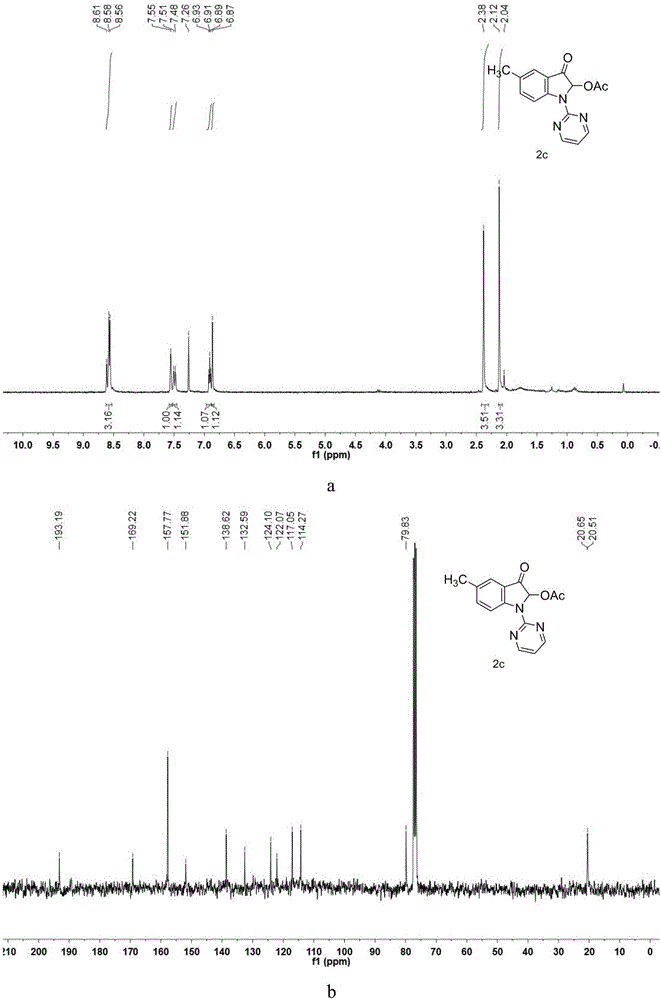
[0049] Synthesis of 5-methyl-2-acetoxy-3-oxo-1-(pyrimidin-2-yl)indole
[0050] Take a 100mL round bottom flask and a suitable size magnet, weigh 5-methyl-1H-indole (5mmol, 755mg) and dissolve it in N,N-dimethylformamide (DMF), and place it in an ice-water bath Slowly add NaH (mass fraction 60%, 7.5mmol, 300mg) for hydrogen extraction reaction, react for 1 hour, transfer to 130°C oil bath, monitor the end of the reaction by TLC after 26 hours, dilute with ethyl acetate and transfer to 250mL for liquid separation The funnel was washed three times with water, and after washing the DMF, the organic phase was collected to remove water with anhydrous sodium sulfate, and the organic solvent was removed by rotary evaporation under reduced pressure to obtain a crude product, which was obtained by column chromatography (ethyl acetate:petroleum ether=2:95 ) to give the product 5-methyl-1H-(pyrimidin-2-yl)-1H-indole reactant. Accurately weigh the 5-methyl-1H-(pyrimidin-2-yl)-1H-indole re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com